1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts
4.7 (545) In stock

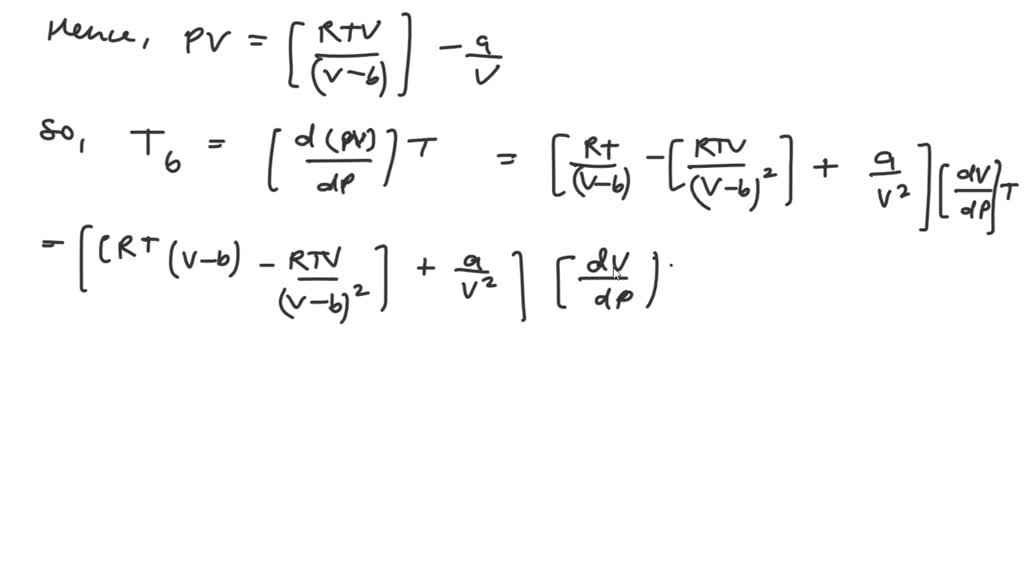
SOLVED: Find the mathematical expression for the Boyle temperature of a van der Waals gas, then find the value of the Boyle temperature of chlorine gas as predicted by the van der

11.2: Intermolecular forces - Chemistry LibreTexts

Chapter 11.1: Real Gases - Chemistry LibreTexts

Derivation of Boyle Temp from real Gas Equation Lecture Note-31 Class XI Chemistry

Van Der Waals Equation of State - an overview

Solved Worksheet: Gas Laws BACKGROUND In this class, we

For a van der Waal's gas, determine Boyle Temperature. [ given mathrm{a}=4.5 mathrm{atm} mathrm{L}^2mathrm{mol}^{-2}, mathrm{b}=0.9 mathrm{L} mathrm{mol}^{-1} and R=0.082 mathrm{L} mathrm{atm} mathrm{K}^{-1} mathrm{mol}^{-1}].609.8K6.09K273K60.98K
Course: Chemistry

Full PDF, PDF, Cell Nucleus
Compressibility Factor Calculator
If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as
a) A gas at 250 K and 15 atm has a molar volume 12 per cent
Temperature reduced, compressibility factor - Big Chemical Encyclopedia
Compressibility factors of air using improved virial equation and P-R
 KOZZI Boxer Briefs - True Black
KOZZI Boxer Briefs - True Black Women Yoga Pants Organic Cotton - Black/Grey
Women Yoga Pants Organic Cotton - Black/Grey Women's Suit 3 Piece Striped Skirt Suit Petite Business Work Suit Formal Wedding Lady Tuxedo(Jacket+Vest+Skirt)
Women's Suit 3 Piece Striped Skirt Suit Petite Business Work Suit Formal Wedding Lady Tuxedo(Jacket+Vest+Skirt) Juzo Compression sleeves - Juzo
Juzo Compression sleeves - Juzo- LIVI Wireless Medium-Impact Wicking Sports Bra
- Gaiam Restore Strength and Flexibility Kit, Multi

