If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as
4.9 (260) In stock


If Z is a compressibility factor, van der Waals equation at low pressure ..

SOLUTION: Dpp 7 gaseous state and chemical energetics - Studypool

If Z is a compressibility factor, van der Waals equation at low pressure ..
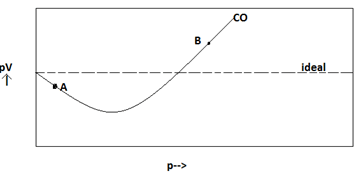
For one mole of a van der Waals gas when b0andT300K the PVvs1Vplot is shown below The value of the van der Waals constant aatmL2mol2is

SOLVED: The fugacity of a van der Waals gas can be determined using the expression for the compressibility factor Z. The expression for fugacity of a van der Waals gas is given

If Z is a compressibility factor, van der Waals' equation at low pressure can be written as
Gaseous State - 2 Free MCQ Practice Test with Solutions - Chemistry

20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
The compression factor (compressibility factor) for one mole of a van der Waals' gas at 0°C - Sarthaks eConnect

012 IfZ is a compressibility factor, van der Waals equation low pressure can be written as: [2014] RT I-끔 (C) Z-I+ Z=1+ (B) Ζ=I.RT (D) Z=l- _ pb VRT

JEE Advanced, Mathematics, Study Material

Jee main-2014-solution-code-h-english
If Z is a compressibility factor, van der Waals equation at low pressure can be written as:a)b)c)d)Correct answer is option 'D'. Can you explain this answer? - EduRev JEE Question
Summary of Equations used to evaluate compressibility factor, z
Compressibility factor (gases) - Citizendium
PPT - GASES PowerPoint Presentation, free download - ID:2088317
 Panache Andorra Full Cup Wired Bra, Simply Be
Panache Andorra Full Cup Wired Bra, Simply Be Double Shoulder Brace Adjustable Sports Shoulder Support Belt Back Pain Relief Double Bandage Cross Compression Shoulder Strap
Double Shoulder Brace Adjustable Sports Shoulder Support Belt Back Pain Relief Double Bandage Cross Compression Shoulder Strap Irresistible Owambe Styles In Burnt Orange Colour Orange formal dresses, Orange bridesmaid dresses, Burnt orange bridesmaid dresses
Irresistible Owambe Styles In Burnt Orange Colour Orange formal dresses, Orange bridesmaid dresses, Burnt orange bridesmaid dresses SPANX, Intimates & Sleepwear, Nwt Spanx Brallelujah Adjustable Plunge Wireless Lift Bra In Toasted Oatmeal
SPANX, Intimates & Sleepwear, Nwt Spanx Brallelujah Adjustable Plunge Wireless Lift Bra In Toasted Oatmeal Miggo Pictar Smart Grip for iPhone and Android [REVIEW]
Miggo Pictar Smart Grip for iPhone and Android [REVIEW] Casual Womens Capri Pants, Linen Trousers, 3/4 Linen Pants, Capris Trousers, Plus Size Trousers Drop Crotch Pants Women Japanese Style Pants
Casual Womens Capri Pants, Linen Trousers, 3/4 Linen Pants, Capris Trousers, Plus Size Trousers Drop Crotch Pants Women Japanese Style Pants