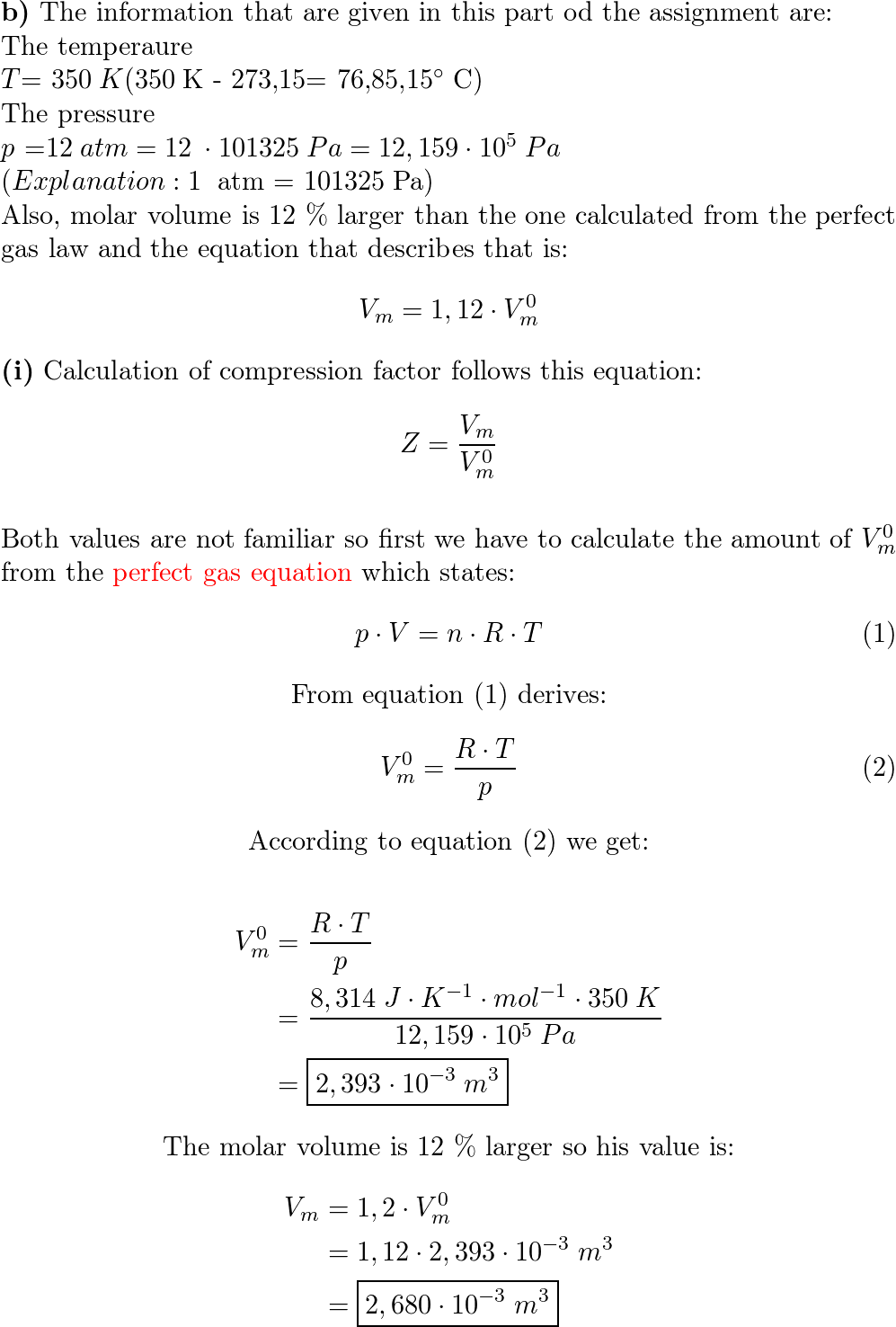
a) A gas at 250 K and 15 atm has a molar volume 12 per cent
4.7 (137) In stock

Chemistry 105, Chapter 5 Exercises

Gas Laws - Chemistry
The pressure exerted by 12 g of an ideal gas at temperature t(in
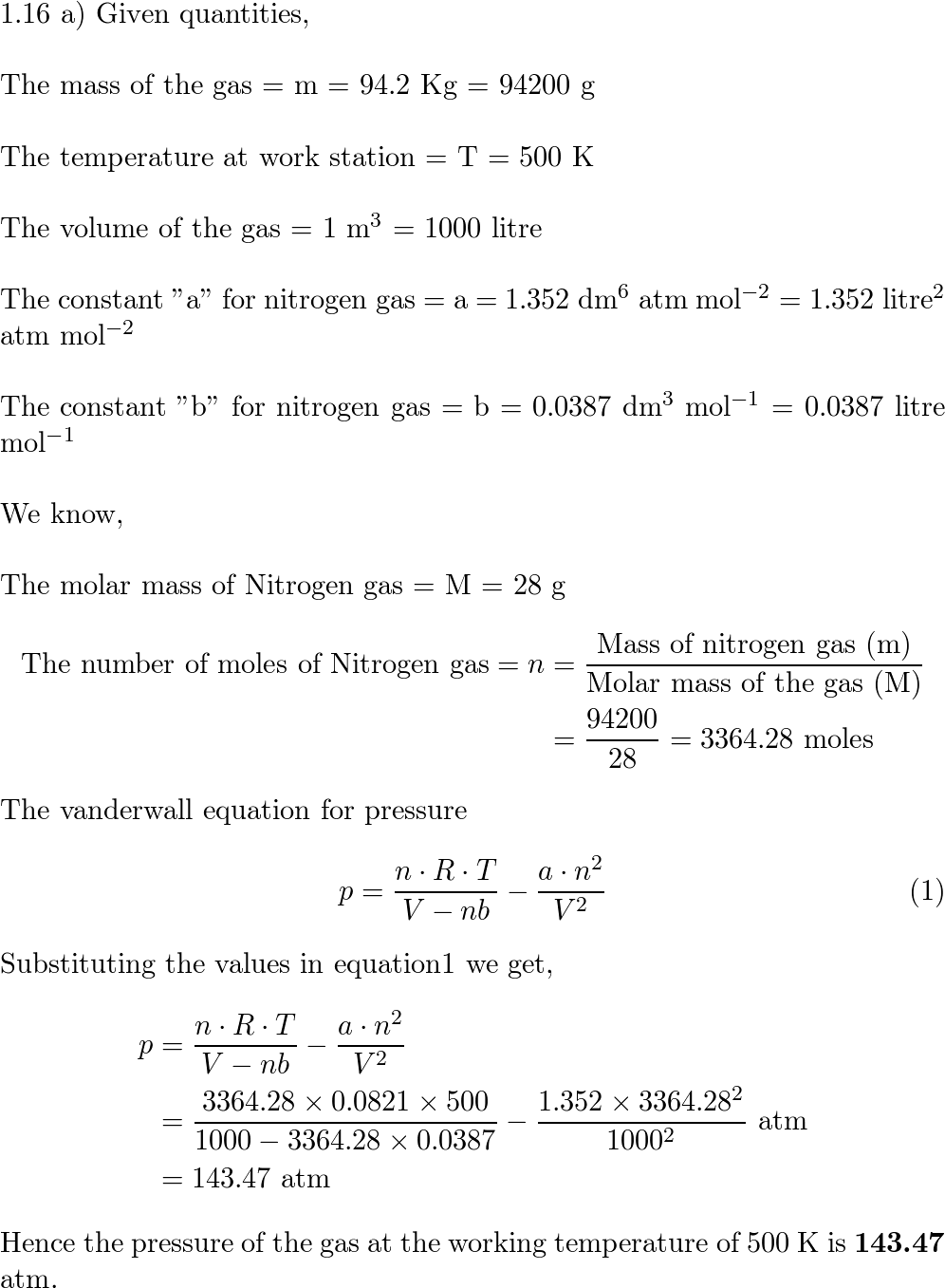
a) In an industrial process, nitrogen is heated to 500 K at

First Law of Thermodynamics - Practice Problems

Solved E1C.3(a) A gas at 250 K and 15 atm has a molar volume

This picture represents a sample of gas at a pressure of 1 atm, a

Experiment 2: Molar Volume of Oxygen

Gas Stoichiometry - Chemistry

Atkins' Physical Chemistry [11th ed.] 0198769865
Compressibility Factor from Redlick-Kwong Equations
Real Gas Behavior The Compression Factor (Z) [Example #2]
The compressibility factor a real gas high pressure is:-1 - frac
Which of the following statements is/are correct? (a) all real
 Pet of the week is a goat named Blitzen - The San Diego Union-Tribune
Pet of the week is a goat named Blitzen - The San Diego Union-Tribune European Sankey Beer Keg Pump - Regular Lever Handle - 8 Steel Barrel
European Sankey Beer Keg Pump - Regular Lever Handle - 8 Steel Barrel Click4she Plus Size Printed Padded Bra For Women and ladies
Click4she Plus Size Printed Padded Bra For Women and ladies Better Choics Women Cotton Air Bra For Women Full Coverage Ultra
Better Choics Women Cotton Air Bra For Women Full Coverage Ultra Gama Alta Ultimate Lift Stage 3 Faja Curvy Support
Gama Alta Ultimate Lift Stage 3 Faja Curvy Support- Is it normal for one breast to bigger than the other? Yep. #themoreyou
