Solved RT B 2. The compressiblity factor for a gas is
4.7 (213) In stock

Answer to Solved RT B 2. The compressiblity factor for a gas is

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks
When does real gas behave as ideal gas? - Quora

Lecture 4-Real-Gases, PDF, Gases

Find the isothermal compressibility `x` of a Van der Walls gas as a function of volume
Slope of graph of compressibility factor(Z) with pressure(P) for hydrogen gas at any pressure i

Non-Ideal Gas Behavior Chemistry: Atoms First

Which of the following statements is/are correct? (a) all real gases are less compressible
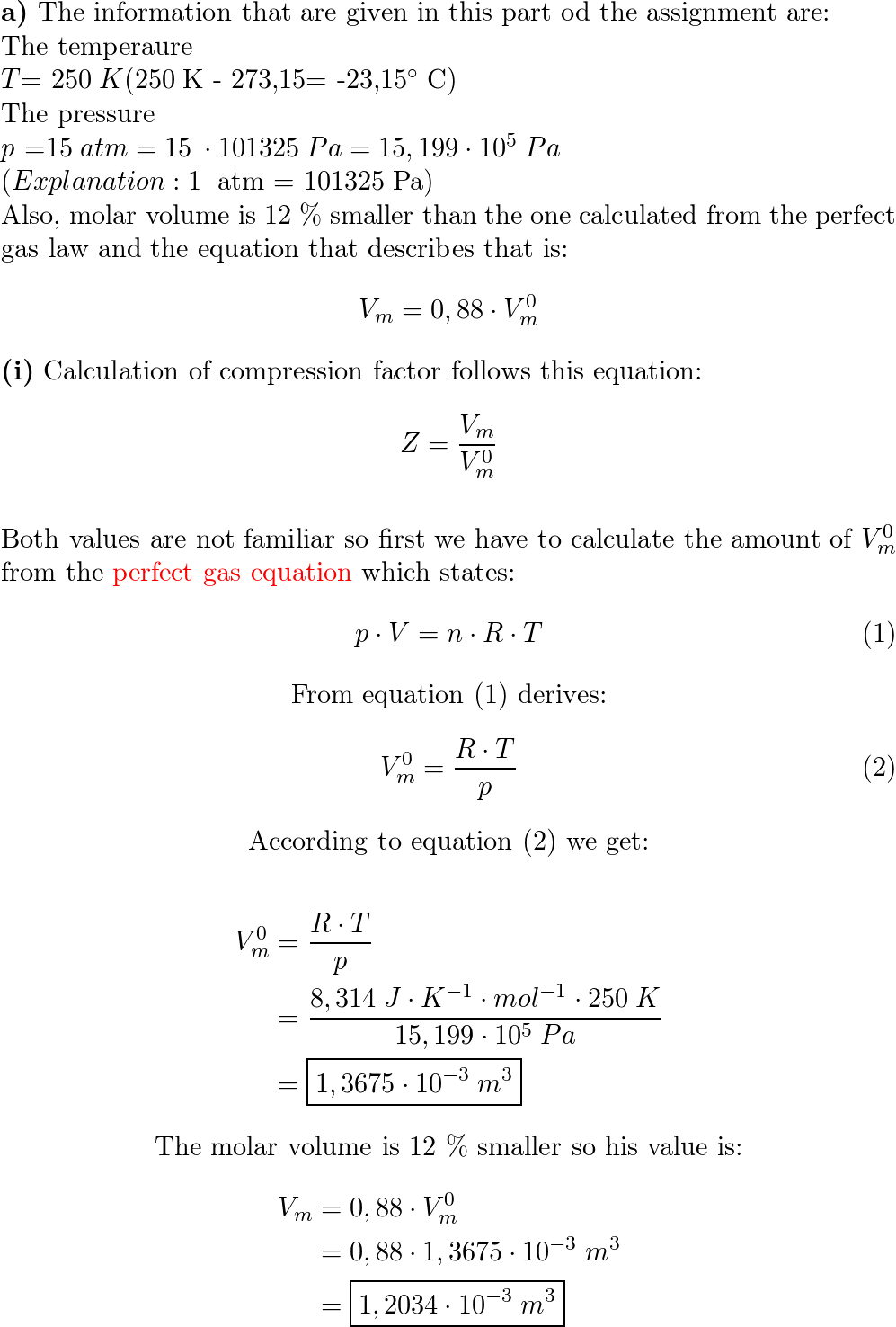
a) A gas at 250 K and 15 atm has a molar volume 12 per cent
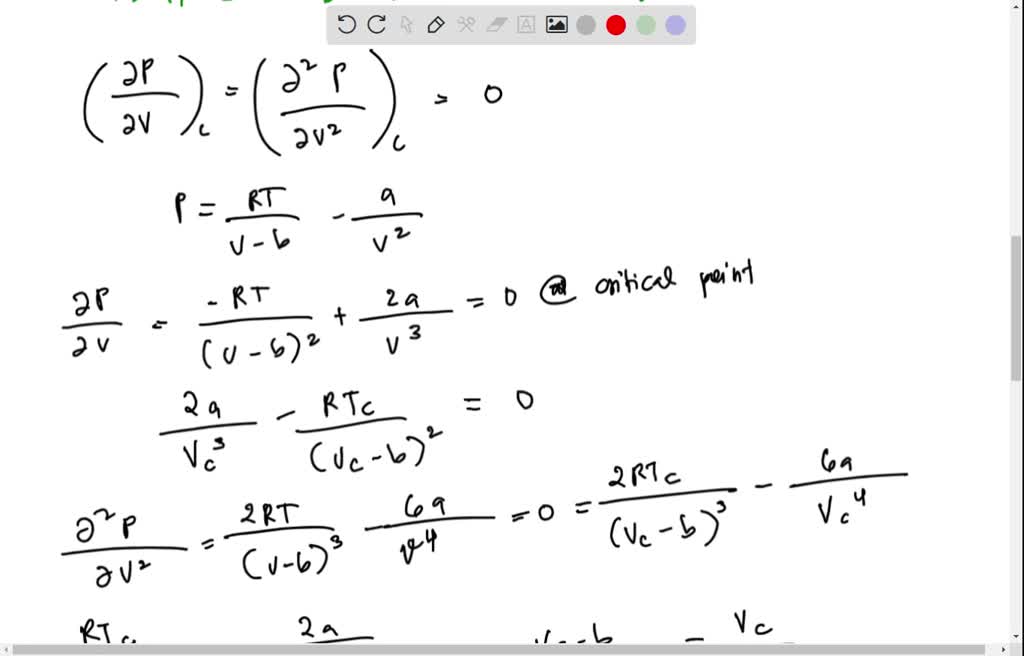
SOLVED: 1) Estimate/ Calculate the critical constants (pc, Vc, and
At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is
Solved We showed, for a van der Waals gas, that the
The compressibility factor a real gas high pressure is:-1 - frac
 Winchester Super X High Brass Game Load 12 Gauge 2.75 1-1/4 oz 7.5 Lead Shot - CASE - Simmons Sporting Goods
Winchester Super X High Brass Game Load 12 Gauge 2.75 1-1/4 oz 7.5 Lead Shot - CASE - Simmons Sporting Goods Lululemon Another Mile Jacket, Women's Fashion, Coats, Jackets and
Lululemon Another Mile Jacket, Women's Fashion, Coats, Jackets and Right Back At It Foil Leggings
Right Back At It Foil Leggings TOP 10 BEST Nursing Bra in Dallas, TX - Updated 2024 - Yelp
TOP 10 BEST Nursing Bra in Dallas, TX - Updated 2024 - Yelp Starline Grendel Basic and 6.8 Basic Straight-Walled Brass -The Firearm Blog
Starline Grendel Basic and 6.8 Basic Straight-Walled Brass -The Firearm Blog Parfait Images – Browse 51,871 Stock Photos, Vectors, and Video
Parfait Images – Browse 51,871 Stock Photos, Vectors, and Video