The compressibility factor a real gas high pressure is:-1 - frac
4.5 (316) In stock

Click here:point_up_2:to get an answer to your question :writing_hand:the compressibility factor for a real gas at high pressure is
Click here👆to get an answer to your question ✍️ The compressibility factor a real gas high pressure is-1 - frac-Pb- -RT-1 - frac -RT- -Pb-11 - frac -Pb- -RT

Real Gas - Definition and Detailed Explanation with FAQs, Compressibility Factor for a Real Gas

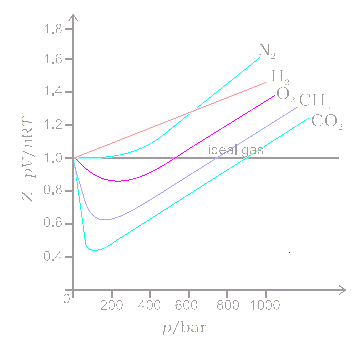
Description of real gases: Compression factor

Compressibility factor (z): real gases deviate from ideal behav-Turito

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics
Why compressibility factor of areal gas is greater than unity at high pressure and temperature? - Quora
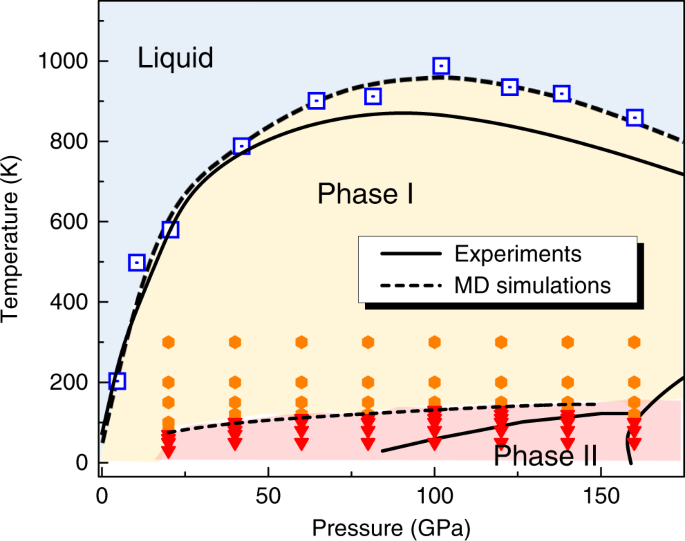
Understanding high pressure molecular hydrogen with a hierarchical machine-learned potential
Van der waals equation: Derivation, Explanation

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Compressibility factor (gases) - Knowino

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

Compressibility factor - Wikipedia

The compressibility factor Z a low-pressure range of all gases except hydrogen is:Z=(1+ displaystylefrac{a}{V_{m}RT})Z=(1 -displaystylefrac{a}{V_{m}RT})Z=(1+displaystylefrac{Pb}{RT})Z = ( 1 - displaystylefrac{Pb}{RT})

Real Gases Introductory Chemistry

Real gas z-Factor chart [2] Download Scientific Diagram
Compressibility Factor Calculator
What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0
 FIORIO MILANO man costume boxer blue cashmere fantasy art FIP BXR 25352 S78 01351 100% nylon polyamide MADE IN ITALY
FIORIO MILANO man costume boxer blue cashmere fantasy art FIP BXR 25352 S78 01351 100% nylon polyamide MADE IN ITALY The Preppy Style Aesthetic: How To Dress Preppy In 2024
The Preppy Style Aesthetic: How To Dress Preppy In 2024 Superdry Ultimate Windbreaker Jacket
Superdry Ultimate Windbreaker Jacket- Conjunto De Ropa Deportiva Casual De Mujer De Moda Coreana 2PCS Traje Deportivo De Manga Larga Para Dama Top E Inferior
 La Senza Lime Green T-Shirt Bra 34B
La Senza Lime Green T-Shirt Bra 34B Women's Silksworth Hooded Down Insulated Jacket - Green
Women's Silksworth Hooded Down Insulated Jacket - Green
