The compressibility factor is Z = PV/R_g T. Evaluate
4.6 (761) In stock

Answer to The compressibility factor is Z = PV/R_g T. Evaluate

Guggenheim's Rule and the Enthalpy of Vaporization of Simple and Polar Fluids, Molten Salts, and Room Temperature Ionic Liquids
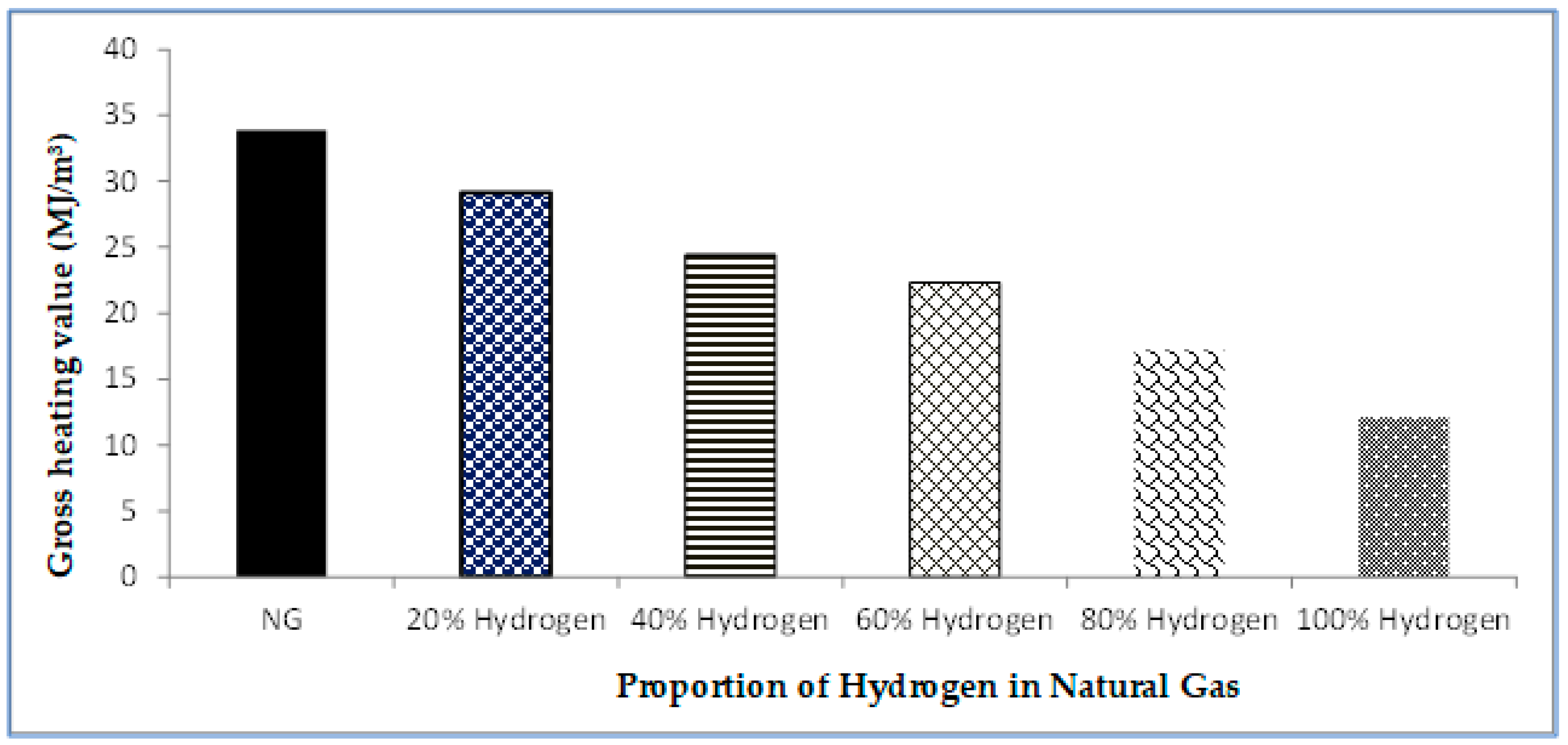
Gases, Free Full-Text
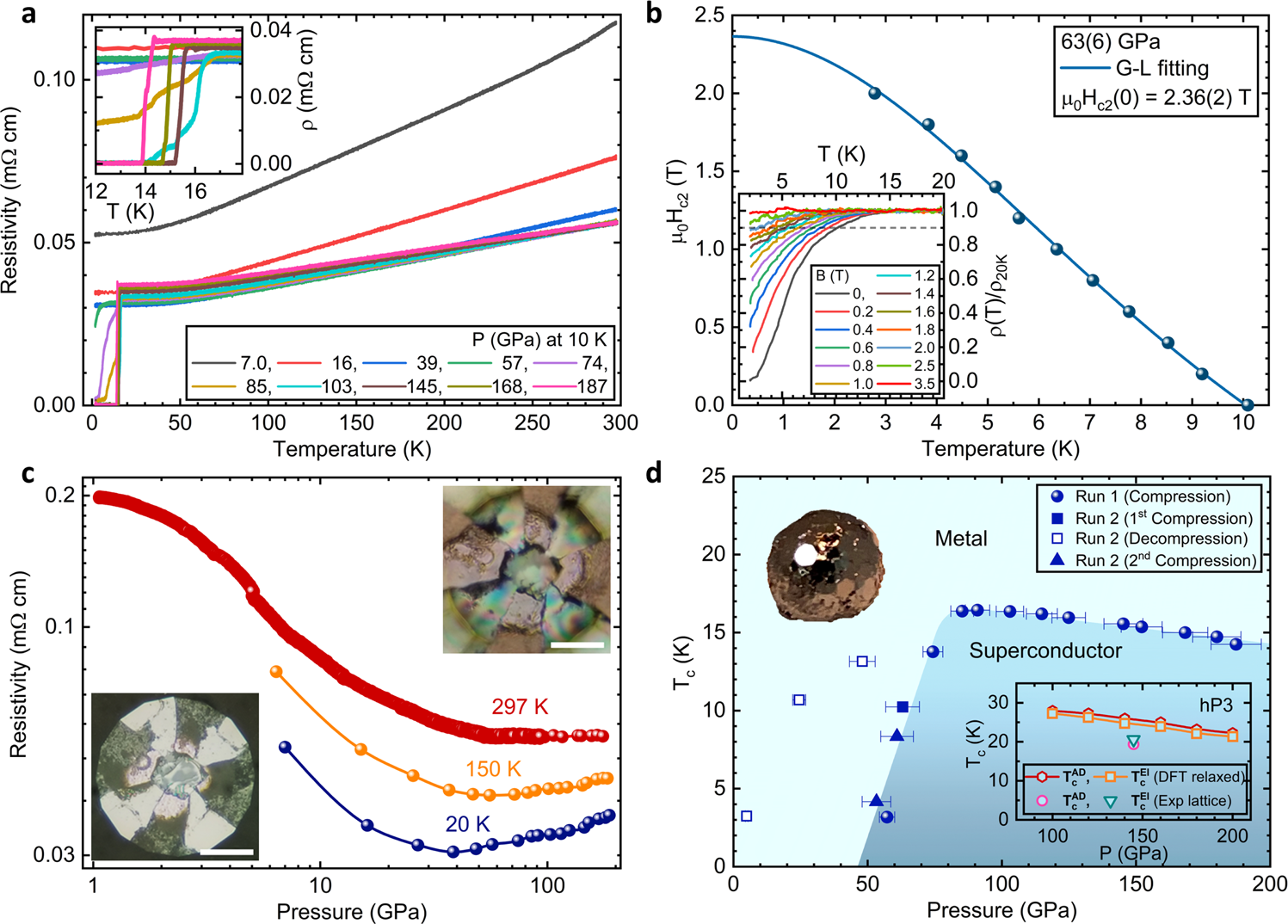
Creating superconductivity in WB2 through pressure-induced metastable planar defects

Compressibility Factor Z Important Concepts and Tips for JEE Main

The given graph represent the variations of Z (compressibility factor (Z)=dfrac {pV}{nRT}) versus P, three real gases A, B and C. Identify the only incorrect statement.For the gas B, b=0 and its

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

Compressibility Factor Calculator - File Exchange - MATLAB Central

plotting - How to plot Compressibility factor Z vs Pressure P using ParametricPlot? - Mathematica Stack Exchange

Physical Chemistry The Compression Factor (Z) [w/1 example]

The compressibility factor of a gas is defined as Z=P V / R T. The compressibility factor of idea
Determine Compressibility of Gases
Cubic Equation of State for the Compressibility Factor - Wolfram
Non Ideal Gas Behavior-chemistry - Non Ideal Gas Behavior
Solved RT B 2. The compressiblity factor for a gas is
000559 Calculation of Compressibility Factor from Redlich-Kwong Equation
 Buy Front Open Butterfly Back Push Up Bra and Panty Set (Red) at
Buy Front Open Butterfly Back Push Up Bra and Panty Set (Red) at Forever Yours High Waist Pants In Fuchsia • Impressions Online
Forever Yours High Waist Pants In Fuchsia • Impressions Online Womens Sexy Bra Underwear Bralette Crop Female Bra Large Top
Womens Sexy Bra Underwear Bralette Crop Female Bra Large Top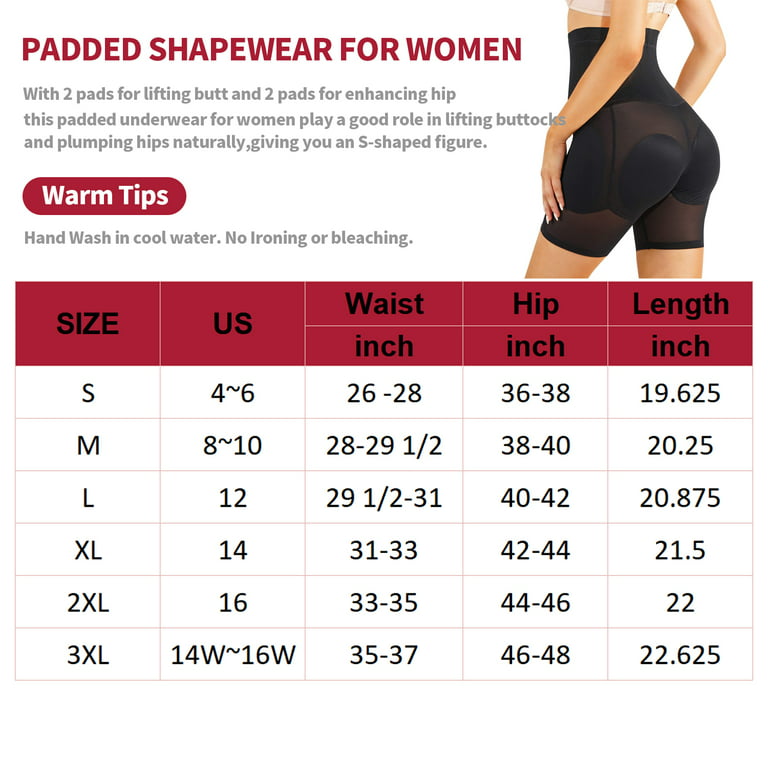 MISS MOLY Womens Shapewear Padded Butt Lifter High Waist Trainer Tummy Control Panties Hip Enhancer with Removable 4 Pads
MISS MOLY Womens Shapewear Padded Butt Lifter High Waist Trainer Tummy Control Panties Hip Enhancer with Removable 4 Pads- What is Anti-Racism? Community Service Center
 Women Push-Up Butt Lifting High Waist Stretchy Skinny Jeans Casual Denim Pants Ladies Zipper Button Jegging Pencil Pants
Women Push-Up Butt Lifting High Waist Stretchy Skinny Jeans Casual Denim Pants Ladies Zipper Button Jegging Pencil Pants
