What is the compressibility factor (Z) for 0.02 mole of a van der Waal
4.9 (111) In stock

(d) (0.1+(1000xx(0.02)^(2))/(V^(2)))V=20xx0.02 =0.1V^(2)-0.4V+0.4=0 =V^(2)-4V+4=0 implies" "V=2L Z=(PV)/(nRT)=(0.1xx2)/(20xx0.02)=0.5

Filo Student Questions For CBSE , Grade 9

Answered: Question 2: For the following parts,…

Full article: Efficient Phase Equilibrium Calculation for
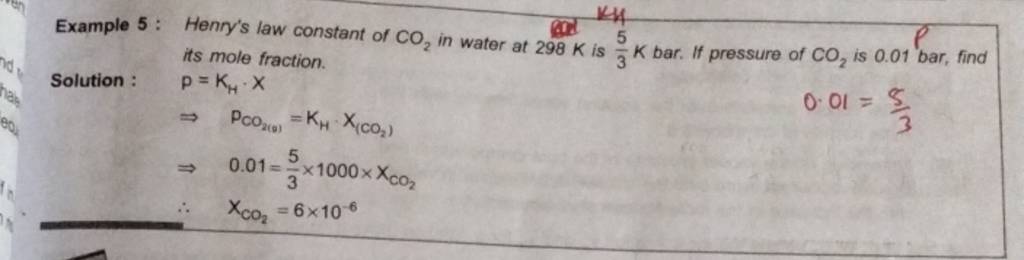
its mole fraction. Solution : P=KH⋅X⇒PCO2( g)=KH⋅X(CO2)⇒0.01=35×100..

its mole fraction. Solution : P=KH⋅X⇒PCO2( g)=KH⋅X(CO2)⇒0.01=35×100..

Non-ideal behavior of gases (article)
Why does CH4 have a greater value of van der Waals' constant than

Investigation of the Properties of Hydrocarbon Natural Gases Under

SOLVED: A certain gas obeys the van der Waals equation with a
Physical Chemistry The Compression Factor (Z) [w/1 example]
Compressibility factor Z - Gaseous State
Is z (compressibility factor) vs P (pressure) graph drawn by
 The Bullet Bra – 1950's Pin-up Necessity
The Bullet Bra – 1950's Pin-up Necessity Buy Rupa Double Layered Non-Wired Full Coverage Bra - White at Rs
Buy Rupa Double Layered Non-Wired Full Coverage Bra - White at Rs Men Quick-Dry Mesh Shorts Gym Fitness Wear Running Basketball
Men Quick-Dry Mesh Shorts Gym Fitness Wear Running Basketball WOMAN MEDIUM LEGGINGS LAVENDER
WOMAN MEDIUM LEGGINGS LAVENDER Let's Get Real — Real BBL Before and After Photos - NOVA Plastic Surgery and Dermatology
Let's Get Real — Real BBL Before and After Photos - NOVA Plastic Surgery and Dermatology PMUYBHF Ladies Underwear Cotton Briefs White Women's Transparent
PMUYBHF Ladies Underwear Cotton Briefs White Women's Transparent