Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
4.8 (167) In stock

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
Compressibility factor Z - PV - nRT is plotted against pressure as shown below-What is the correct order for the liquefiability of the gases shown in the above graph- A- CO 2- CH 4- N 2- H 2B- H 2- CH 4- N 2- CO 2C- CH 4- H 2- N 2- CO 2D- H 2- N 2- CH 4- CO 2
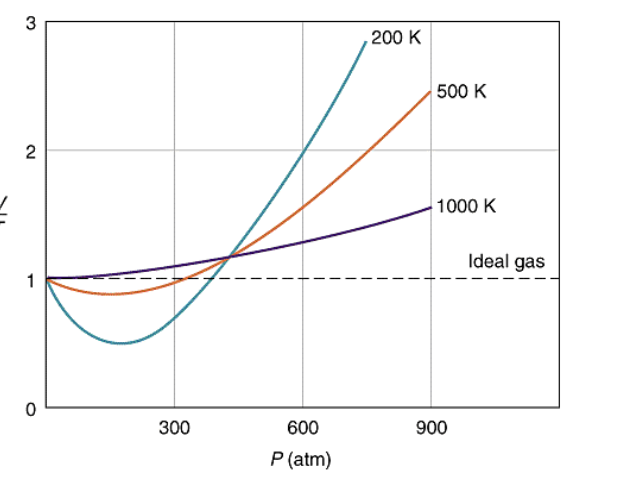
Solved 1. The plot below shows how compressibility factor

Compressibility factor, Z of a gas is given as `Z=(pV)/(nRT)` (i

Compressibility Factor Z Important Concepts and Tips for JEE Main

Compressibility factor Z - Gaseous State

y factor Compressibility factor 2 V is plotted agalnst pressure RT

States of Matter, PDF, Gases

Classplusapp - NEET CHEM-Ch 5, PDF, Gases
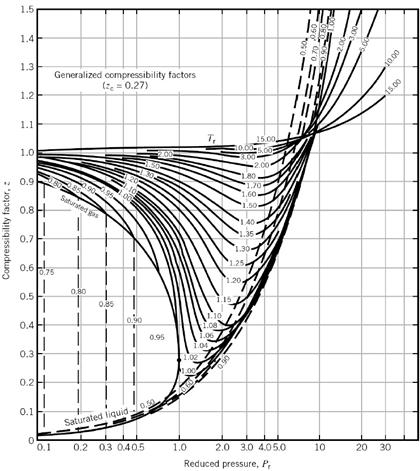
Z= PVm / RT for gases as a function of the reduced
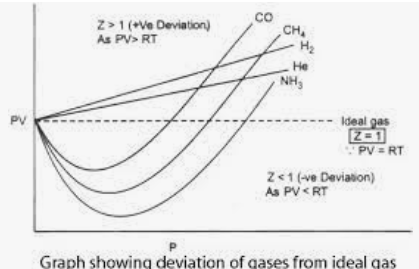
The compressibility factor for an ideal gas is: (A) 1.5 (B) 1.0 (C) 2.0 (D) Infinity
Why can gases with compressibility factor>1 and <1 be liquefied
Consider the graph between compressibility factor Z and pressure P
My publications - CHM 201-LECTURE IV-REAL GASES - Page 8 - Created with Publitas.com
What is the value of compressibility factor in terms of vander





