Compressibility factor (Z) for a van der Waals real gas at
4.8 (714) In stock

Share your videos with friends, family and the world

Deviation of Gas from Ideal Behavior
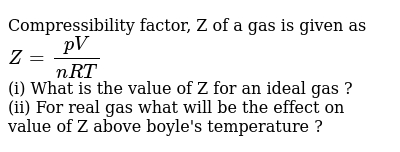
Compressibility factor, Z of a gas is given as Z=(pV)/(nRT) (i) What
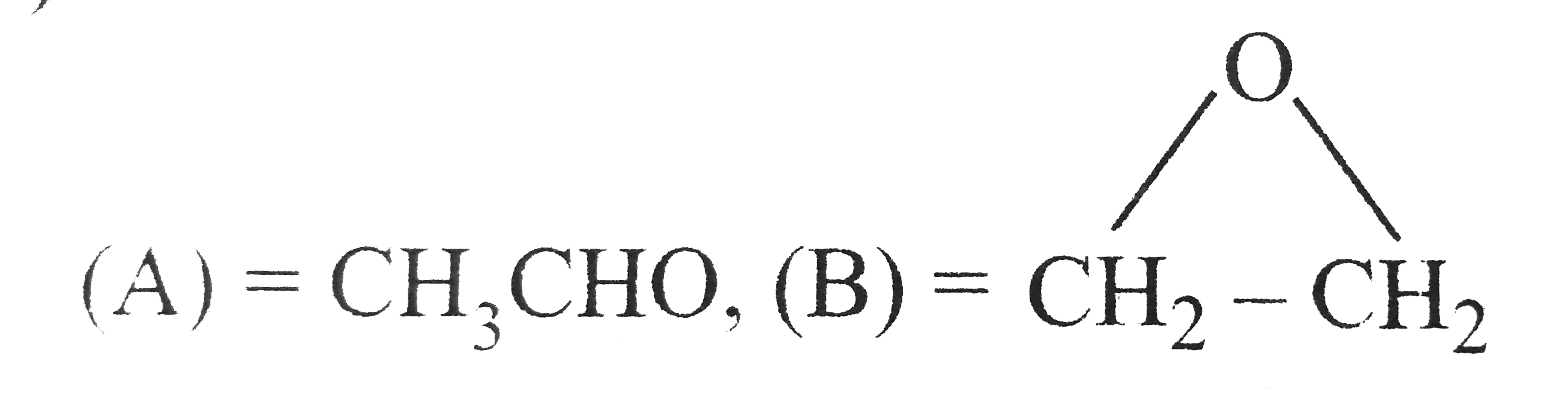
Complete Solutions to Mock Test 1 of chapter MOCK TEST of Class 11 book with complete answers and questions

Non-Ideal Gas Behavior Chemistry: Atoms First

Non-Ideal Gas Behavior Chemistry: Atoms First

Compressibility factor (z): real gases deviate from ideal behav-Turito
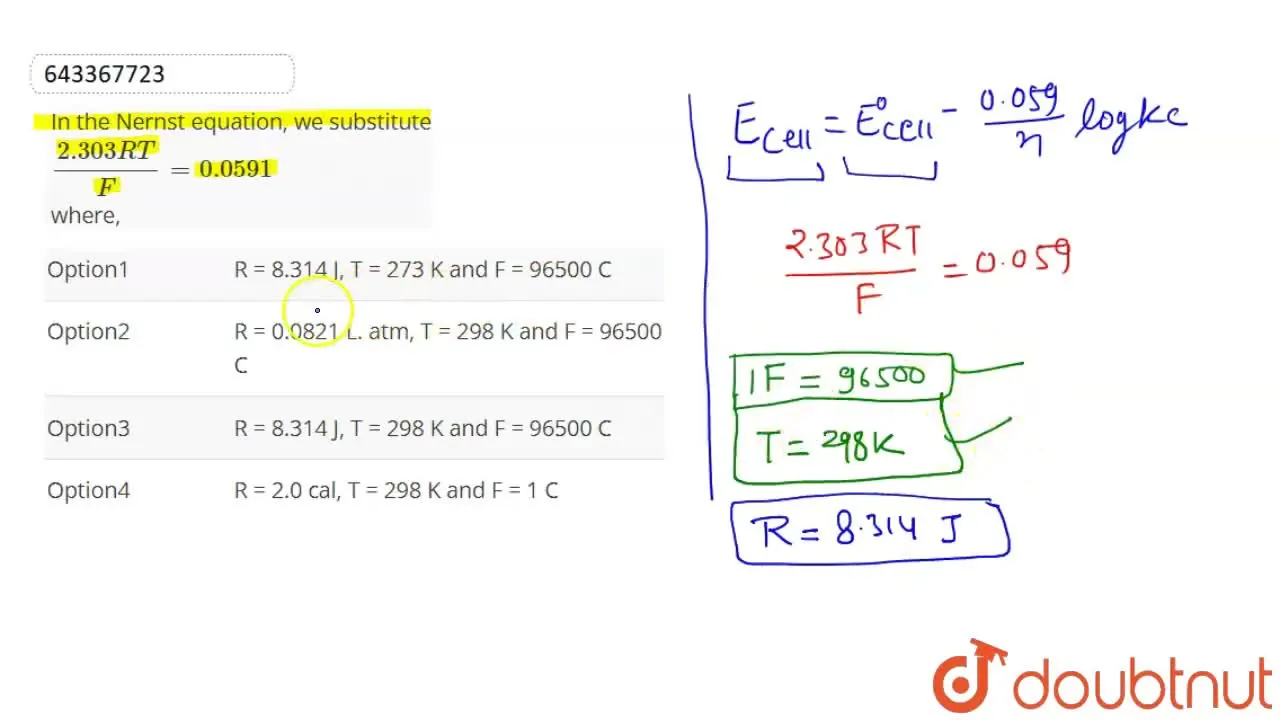
R = 8.314 J, T = 298 K and F = 96500 C

What is the compressibility factor (Z) for 0.02 mole of a van der Waals' gas at pressure of 0.1 a

Answered: Compression factor of a gas with van…
Compressibility Factor - an overview
3.2 Real gas and compressibility factor – Introduction to
Compressibility Factor, z vs Pressure, P (kPa)
e Compressibility factor (Z) for hydrogen WRT pressure and temperature