Pick only the incorrect statement.for gas A, a=0,the
4.6 (666) In stock

Click here:point_up_2:to get an answer to your question :writing_hand:pick only the incorrect statement
Click here👆to get an answer to your question ✍️ Pick only the incorrect statement-for gas A- a-0-the compressibility factor is linearly dependent on pressure-for gas C-aneq 0-bneq 0-it can be used to calculate a and b by giving lowest P value-for gas B-0-if b-0-the compressibility factor is lineraly dependent on pressure-slope all three gases high pressure is positive
Solution- -C-xA0-for gas C-a-x2260-0-b-x2260-0- it can be used to calculate a and b by giving lowest P value-According to the real gas equation-The constants -apos-a-apos- and -apos-b-apos- are Van der Waals constant for attraction and volume for a given gas-The -apos-a-apos- values for a given gas are measure of intermolecular forces of attraction- More are the intermolecular forces of attraction- more will be the value of a-xA0-For a given gas van der Waals constant of attraction -apos-a-apos- is always greater than van der Waals constant of volume -apos-b-apos-xA0-The gas having higher value of -apos-a-apos-xA0- can be liquefied easily and therefore H2 and He are not liquefied easily-According to this- for gas A-Z-gt-1-a-0 and its dependence on P is linear at all pressure and for gas B-Z-lt-1-b-0 and its dependence on P is linear at all pressure-Also- at high pressure- the slope is positive for all real gases

Liquid 116. Which of the following is the only incorrect statement

variations of 2 12.7 (a) eb (c)-(ar (d) - 6. The given graph
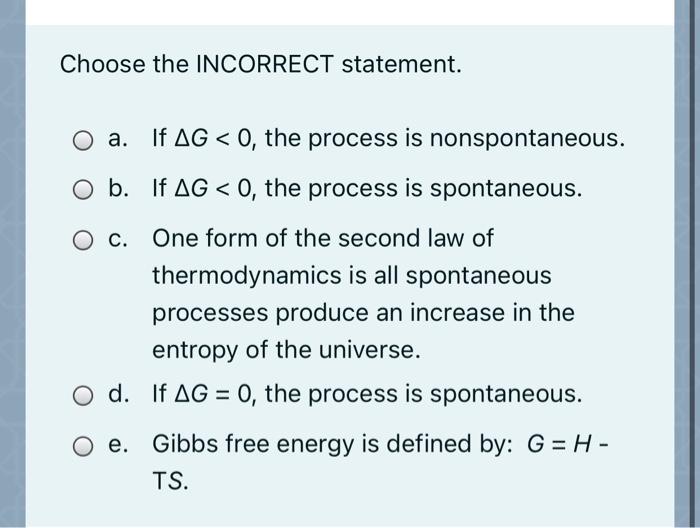
Solved Choose the INCORRECT statement. O a. If AG < 0, the

The given graph represents the variation of Z(compressibility

Solved Select an INCORRECT statement about different states

Pick only the incorrect statement.for gas A, a=0,the

Solved For the reaction: N2(g) + 3H2(g) + 2NH3(g) AH = -92

Solved Which of the following is an incorrect statement

Kevin O'Leary - Wikipedia
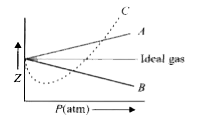
For the gas C which is a typical real gas for which neither a nor b =0
Solved The virial expansion of the compression factor (Z)
How to Calculate Compression Ratio: 9 Steps (with Pictures)
the compression factor one mole of a vander waals gas 0 C and 100 atm pressure is found to be 0.5
Chapter 8 Real Gases. - ppt download
Solved 1. Consider the following gas at a given temperature.
 Buy D'chica Slip-on Strapless Bra for Womens Girls (Pack of 2) Cotton Non-Padded Full Coverage Wire Free Tube Bra for Low-Cut Tops, Dresses, Off Shoulder/Crop Tops Western Outfits Online In India At
Buy D'chica Slip-on Strapless Bra for Womens Girls (Pack of 2) Cotton Non-Padded Full Coverage Wire Free Tube Bra for Low-Cut Tops, Dresses, Off Shoulder/Crop Tops Western Outfits Online In India At- Ever New off shoulder long sleeve mini dress in blue floral
- Zara Clothes Online
- Fluffy Knit Leggings
 Ropa Interior Sin Costuras De Control De Abdomen De La Cintura Alta Para Mujeres Postparto, Calzones Reductores Levantacola, Pantalones Cortos Moldeadores, Mode de Mujer
Ropa Interior Sin Costuras De Control De Abdomen De La Cintura Alta Para Mujeres Postparto, Calzones Reductores Levantacola, Pantalones Cortos Moldeadores, Mode de Mujer Victoria's Secret
Victoria's Secret


