Medical device regulations, classification & submissions
4.9 (671) In stock

Medical device regulations vary in Canada, the U.S. & the EU. Risk-based classification systems determine data requirements for regulatory oversight for medical devices. MaRS

Medical device regulations, classification & submissions

Medical device regulations, classification & submissions

Medical device regulations, classification & submissions
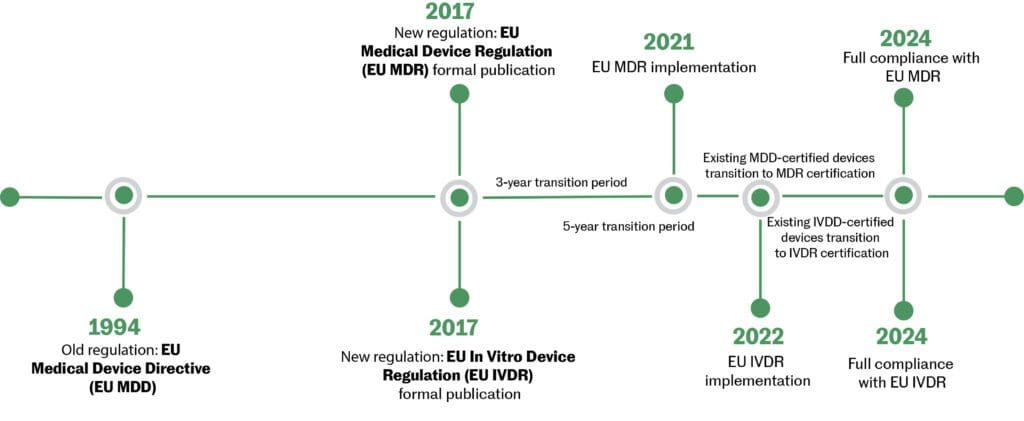
Medical device regulations, classification & submissions

Medical device regulations, classification & submissions
23 Class II division 2 malocclusion
Difference Between Mhc Class 1 and Mhc Class 2 Proteins
Class II Resin Restorations: Quick Tips to Help Manage the “Step
Stability of a severe Class II malocclusion correction in a
Class II, Division 2 Malocclusion, Severe Upper Incisor Retroclination - Case Gallery
- FAQs about our pregnancies 💕 #pregnancyupdate (follow our Patreon for more updates, link in bio)
 49 Vintage For The Future A Norma Kamali Retrospective By What
49 Vintage For The Future A Norma Kamali Retrospective By What Spyder, Underwear & Socks, Spyder Performance Boxer Briefs 2xl Mesh Panels
Spyder, Underwear & Socks, Spyder Performance Boxer Briefs 2xl Mesh Panels Plus Size Crotchless Lingerie Cupless Teddy Bodysuit, Women's Plus Contrast Lace Medium Stretch * Lingerie Teddy Bodysuit
Plus Size Crotchless Lingerie Cupless Teddy Bodysuit, Women's Plus Contrast Lace Medium Stretch * Lingerie Teddy Bodysuit Aster Organic Cotton Wide Leg Pant
Aster Organic Cotton Wide Leg Pant Women's Posture Corrector T-Shirt | Anti Back Pain | Straightens Back and Shoulders | Undershirt | Short Sleeves
Women's Posture Corrector T-Shirt | Anti Back Pain | Straightens Back and Shoulders | Undershirt | Short Sleeves
