Just a few neoantigens may be enough for T cells to control prostate cancer
5 (496) In stock
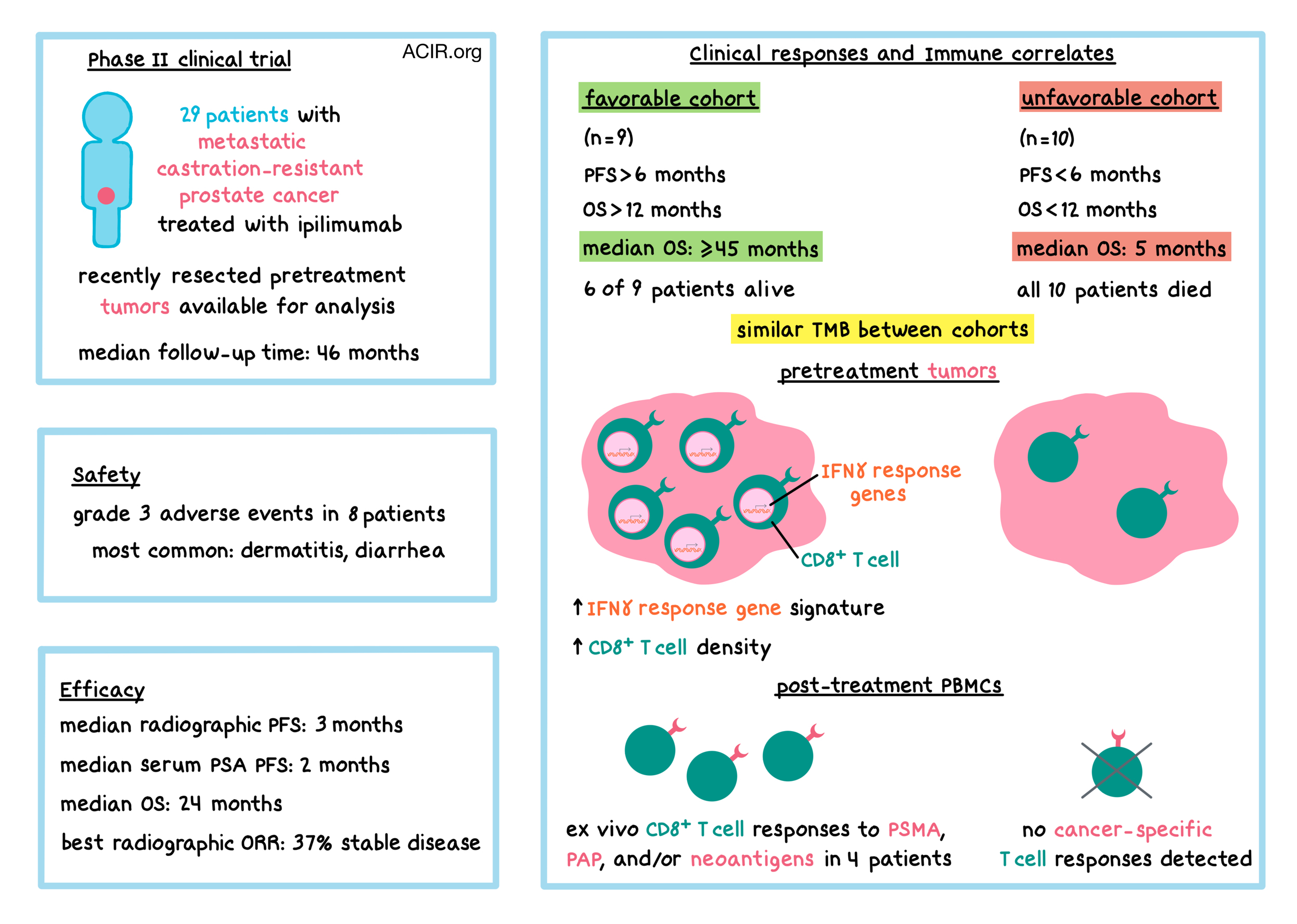
In a phase II clinical trial, 29 patients with metastatic castration-resistant prostate cancer were treated with ipilimumab after tumor resection. Median radiographic PFS was 3 months, median clinical PFS was 2 months, and median OS was 24 months. Best ORR was stable disease in 37% of patients. In the “favorable” cohort (PFS>6 months, median OS of 45 months), pretreatment tumors had increased CD8+ T cell density and IFNγ response gene signature compared with the “unfavorable” cohort (PFS<6 months, median OS of 5 months), while TMB was similar between cohorts. In post-treatment PBMCs, CD8+ T cell responses to PSMA, PAP, and/or neoantigens were found in 4 patients, all of which were in the favorable cohort.
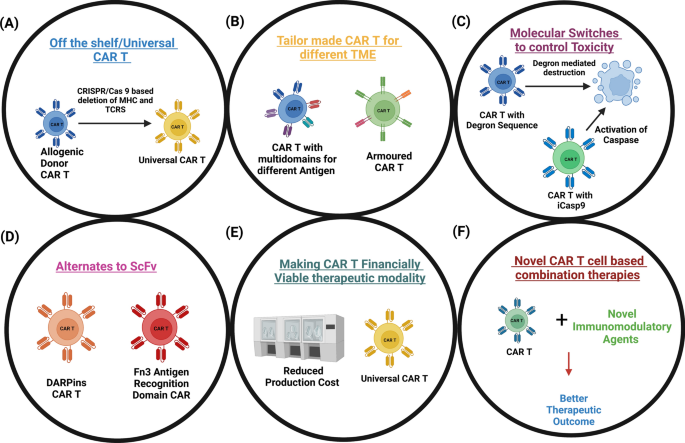
Harnessing the potential of CAR-T cell therapy: progress
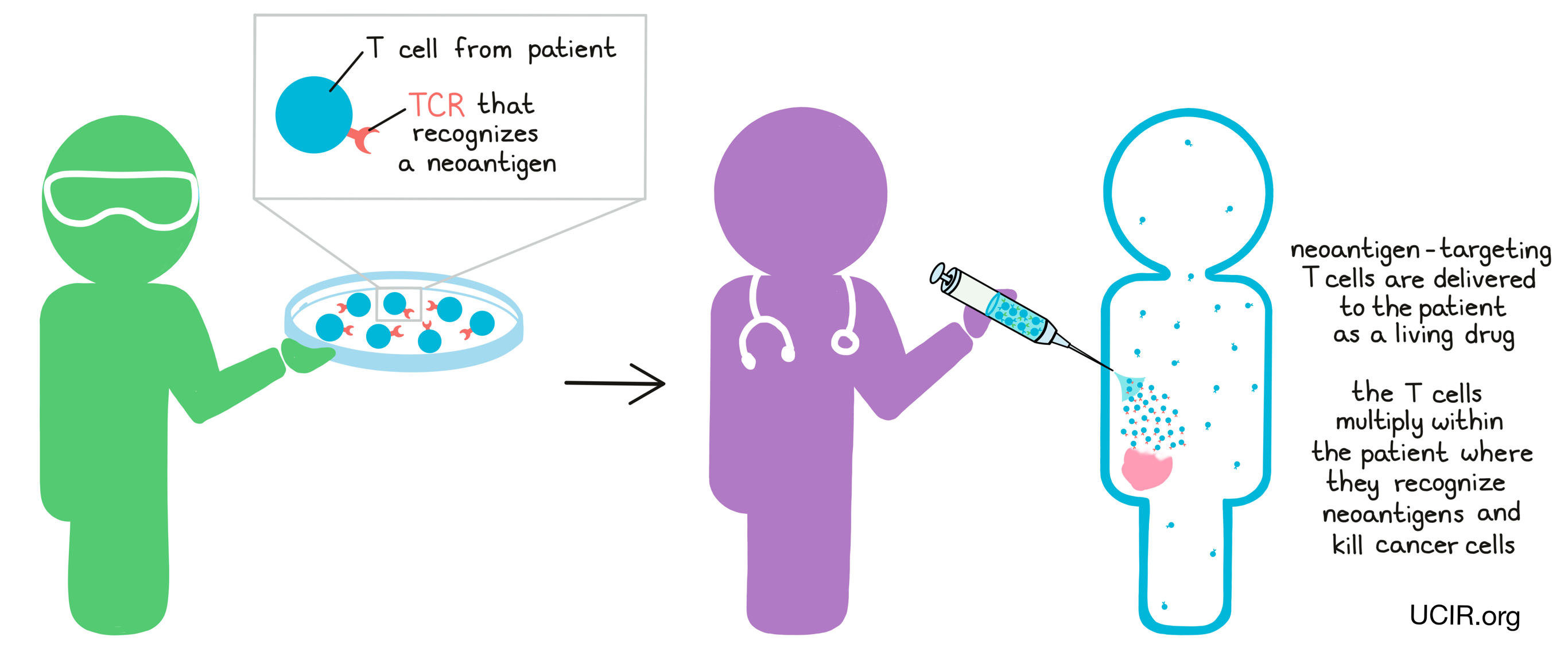
What is neoantigen-based therapy?
Making the cut: exitrons as a new source of neoantigens

Personalized neoantigen-based T cell therapy triggers cytotoxic

Just a few neoantigens may be enough for T cells to control
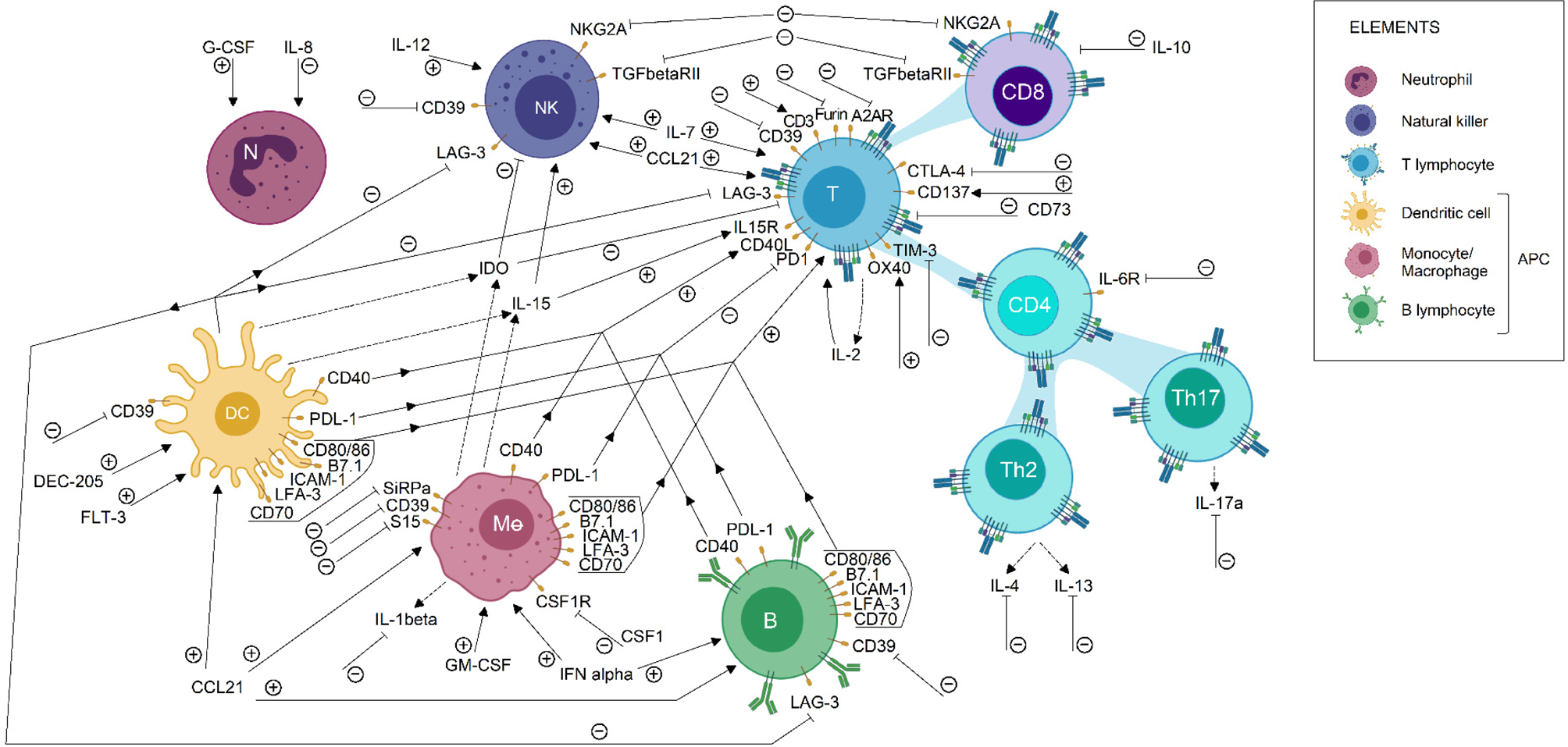
Biological bases of cancer immunotherapy

The role of regulatory T cells in the pathogenesis and treatment

Advanced immunotherapies for glioblastoma: tumor neoantigen
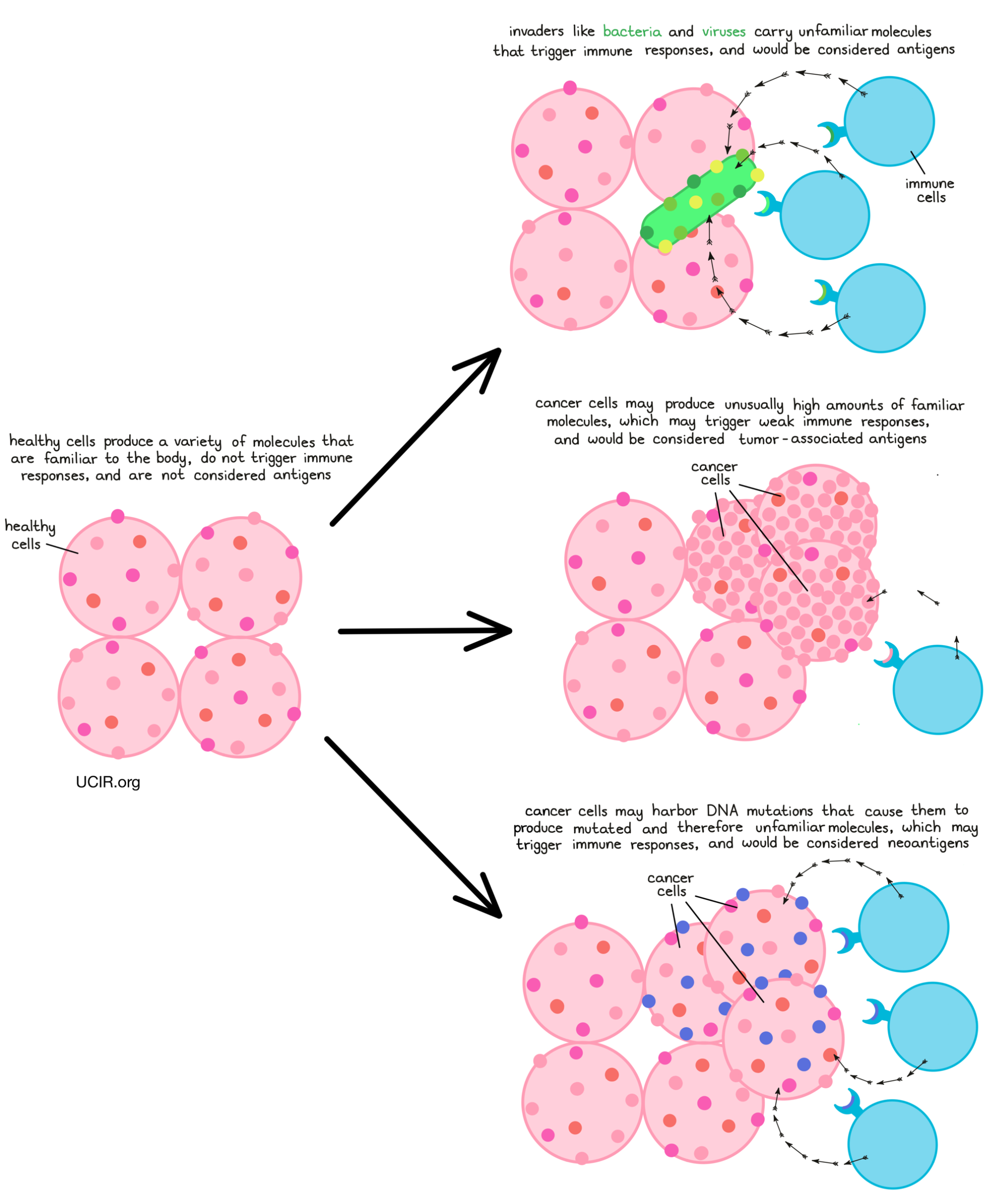
What is neoantigen-based therapy?

Neoantigen responses, immune correlates, and favorable outcomes

Immunotherapy mechanisms in prostate cancer. a Sipuleucel-T
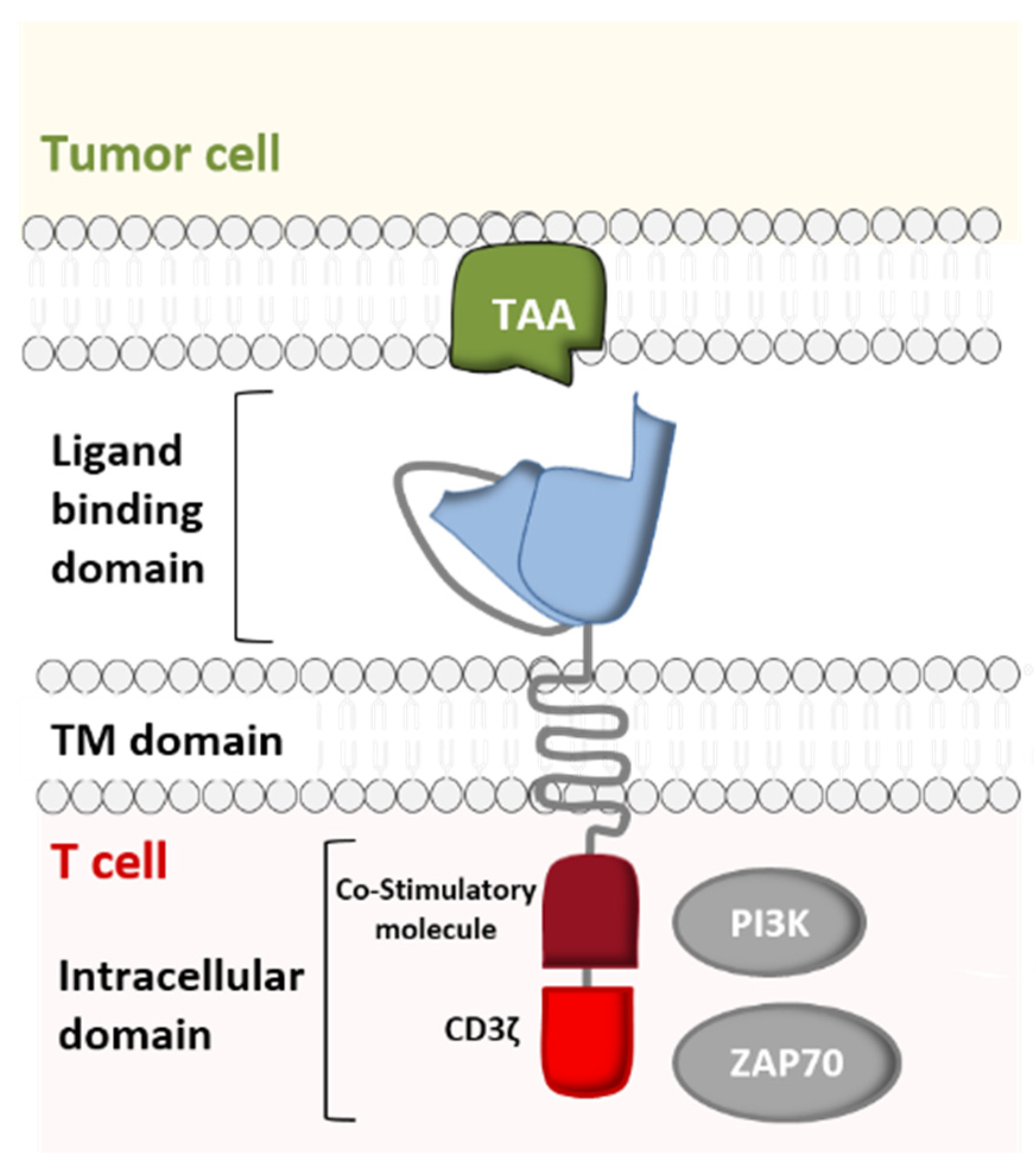
IJMS, Free Full-Text
New Method of Killing Cancer Cells Developed
Eastern Sierra Cancer Alliance – CARE • SUPPORT • EDUCATION • COMMUNITY
Cancer Prevention and Screening: Progress Made and Progress to Come
Octobre rose : la prévention du cancer du sein avec le sport
The myeloproliferative blood cancers—essential thrombocytosis (ET





