The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1)
4.7 (112) In stock
The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1) X 2)X2 3)X4 4)X8
The vapour pressure of a solution having 2-0 g of solute X -gram atomic mass-32 g-mol- in 100 g of CS2 -vapour pressure -854torr- is 848-9 torr-The molecular formula of solute 1- X 2-X2 3-X4 4-X8

Vapour pressure of a solution of `5g`of non-electrolyte in `100g`water at a

Solutions (1-47) - Final, PDF, Solubility
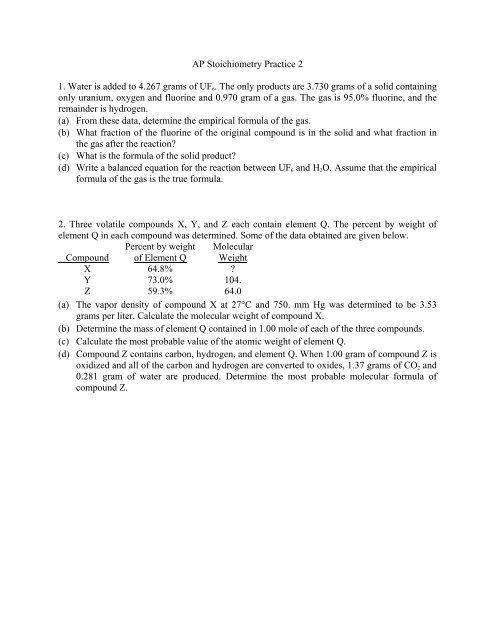
Stoichiometry Practice 2 answer key

our WUSS) 22. The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass = 32 g mol-1) in 100 g of CS, (vapour pressure = 854 torr)

Solutions (1-47) - Final, PDF, Solubility
The vapour pressure of a solution having 2.0 gram of solute X in hundred gram of CS2 is it 848.9 torr. the molecular formula of the solute is

LOPOUL 2. The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass = 32 g mol-') in 100 g of CS, (vapour pressure = 854 torr) is
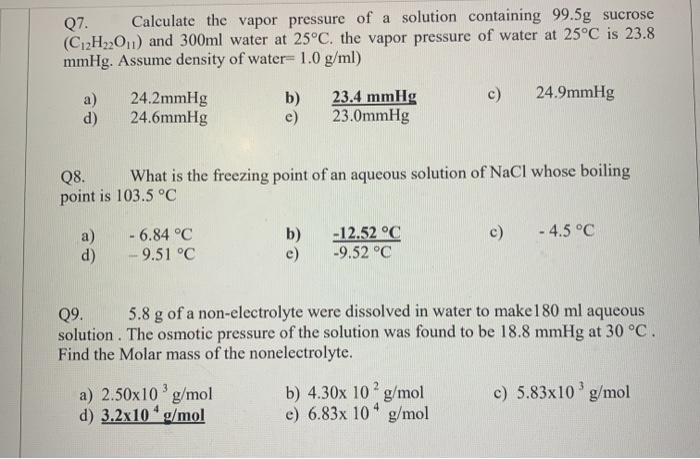
Solved Q1. Complete the followings: (0.5 Mark each) a) If a

The vapour pressure of CS2 50°C is 854 torr and a solution of 2.0 gm sulphur in 100 gm of CS2 has vapour pressure 848.9 torr. If the formula of sulphur molecule
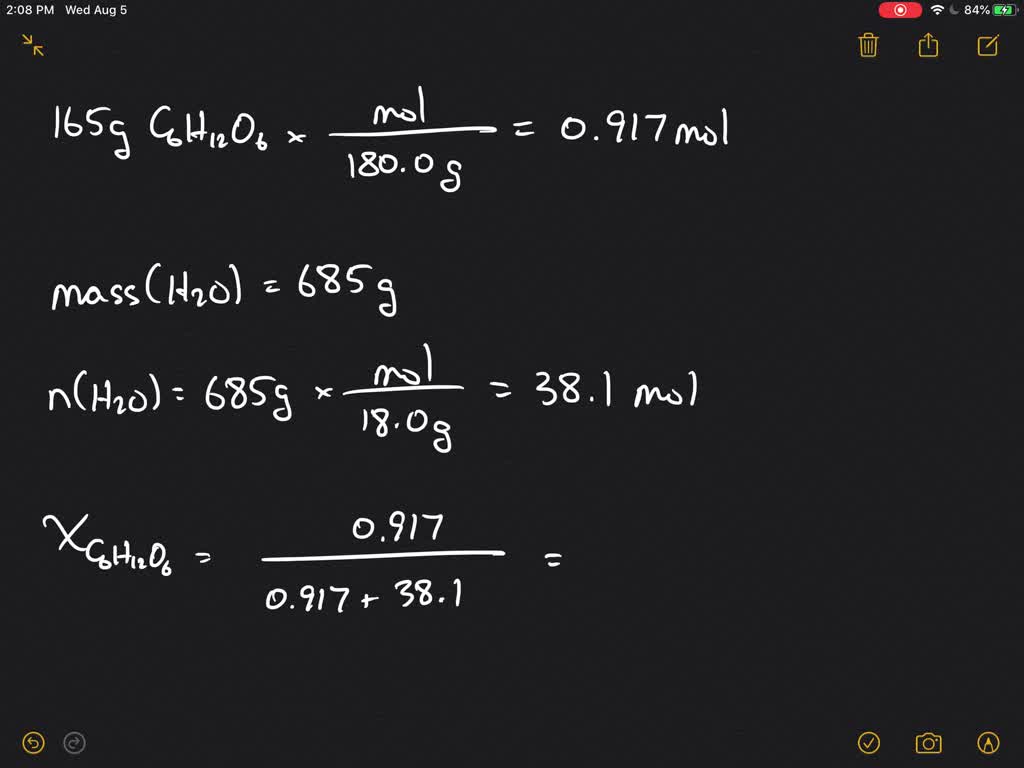
⏩SOLVED:Calculate the vapor pressure at 25^∘ C of a solution…

Solutions (1-47) - Final, PDF, Solubility
HUGGIES Fralda Huggies Supreme Care G - 32 Fraldas
Tablet Vasoun Kids 7 Polegadas Tablet para Crianças Android 11 Tablet 2 Gb Ram 32 Gb
 Rainbow Glitter Look Background | Art Board Print
Rainbow Glitter Look Background | Art Board Print Stay ahead of the game with these high-performance compression workout leggings
Stay ahead of the game with these high-performance compression workout leggings Time and Tru Women's Cargo Capri Pants #Ad #Women, #SPONSORED, #Tru, #Time
Time and Tru Women's Cargo Capri Pants #Ad #Women, #SPONSORED, #Tru, #Time Seamless Lounge Bralette Top for Women XS-XXL
Seamless Lounge Bralette Top for Women XS-XXL Womens Crochet Top Lace Bralette Knit Bra Boho Beach Bikini Halter Cami Crop Top
Womens Crochet Top Lace Bralette Knit Bra Boho Beach Bikini Halter Cami Crop Top Lynn & Linda PRINT AD - 1949 ~ state fair midway ~ high wire ~ aerial act
Lynn & Linda PRINT AD - 1949 ~ state fair midway ~ high wire ~ aerial act