physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange
4.9 (395) In stock

The compressibility factor of a gas is defined as $Z = pV/(nRT)$. If attractive intermolecular forces dominate then $Z$ tends to be smaller than 1, and vice versa if repulsive forces dominate. In

Metal–organic framework - Wikipedia

Sustainability, Free Full-Text
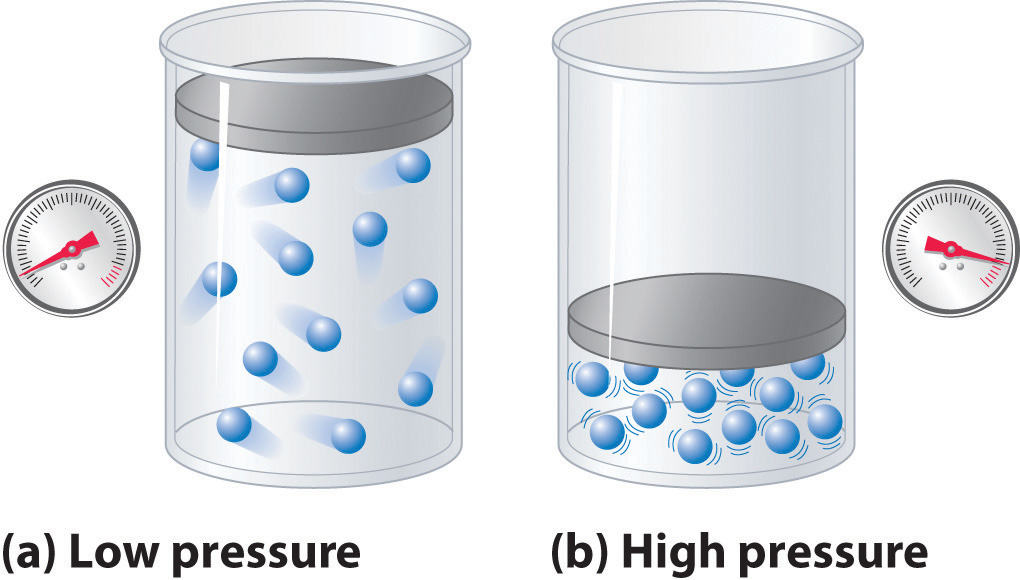
The Behavior of Real Gases
If a volume of water is heated above its critical temperature
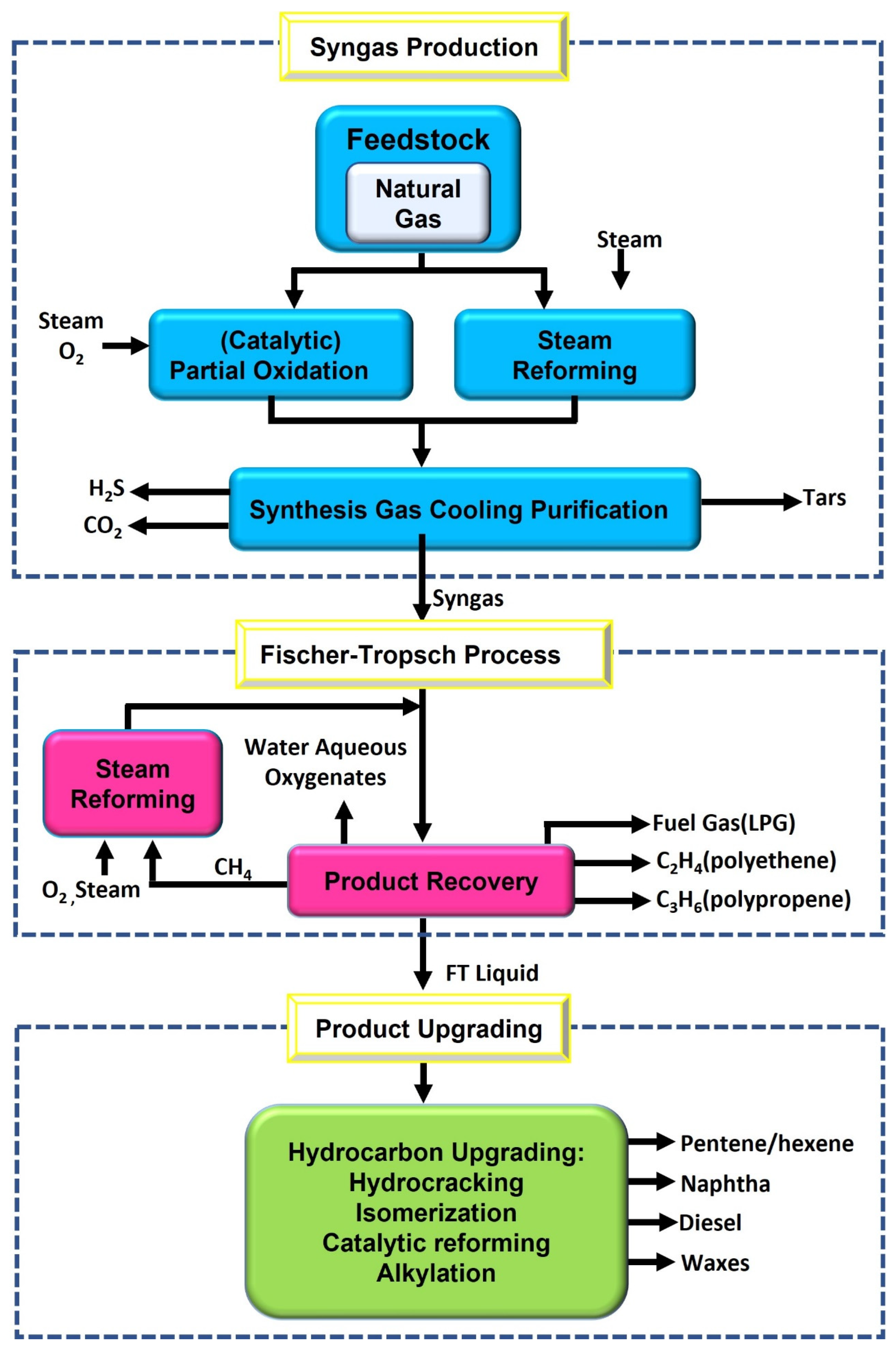
Methane, Free Full-Text

Non-Ideal Gas Behavior Chemistry: Atoms First

/energies/energies-15-05823/article_de
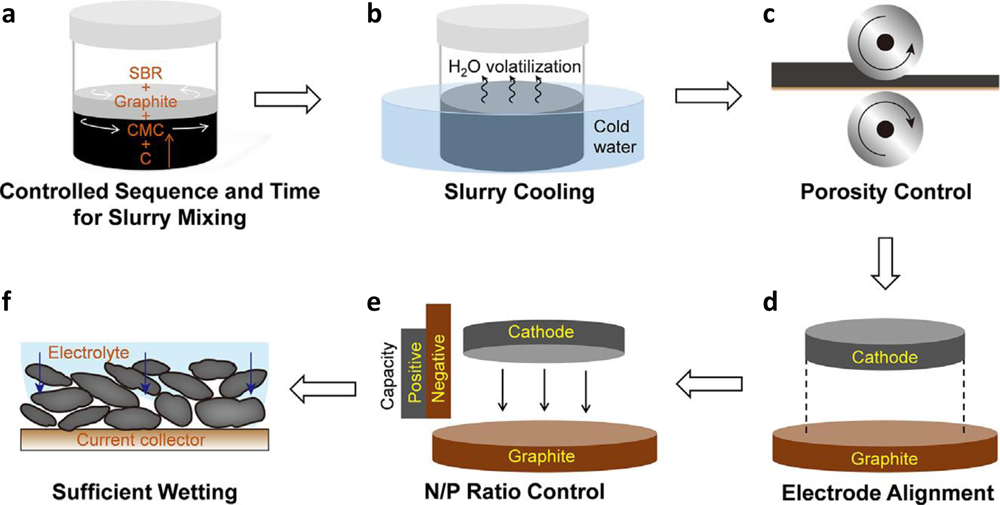
Best practices in lithium battery cell preparation and evaluation

physical chemistry - Pressure vs volume plot for real gas and
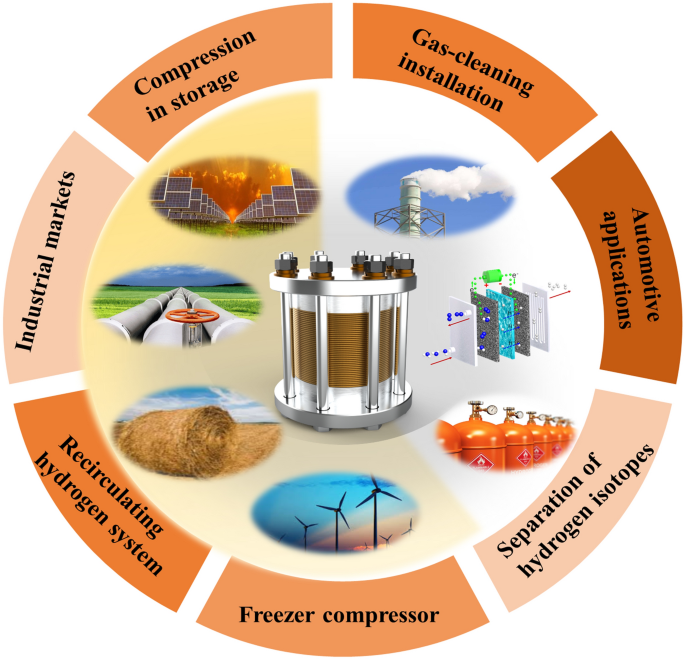
Electrochemical Compression Technologies for High-Pressure
Summary of Equations used to evaluate compressibility factor, z
Compressibility Factor - an overview
What is compressibility factor? What is its value for ideal gas
Compressibility factor - Wikiwand
My publications - CHM 201-LECTURE IV-REAL GASES - Page 8 - Created with Publitas.com
 Push Sticks
Push Sticks Wacoal 38dd Black Soft Embrace Front Close Racerback Underwire Bra 851311 for sale online
Wacoal 38dd Black Soft Embrace Front Close Racerback Underwire Bra 851311 for sale online YOLAI Women Solid Lace Buckle Shapewear Tummy Tucking Body Shaper Underwear Breathable Stretch Control Panties (Beige, S) : : Clothing, Shoes & Accessories
YOLAI Women Solid Lace Buckle Shapewear Tummy Tucking Body Shaper Underwear Breathable Stretch Control Panties (Beige, S) : : Clothing, Shoes & Accessories Fashion Beach Women Lingerie Set Sleepwears G-string Bra (7668) - EasyShopping24x7
Fashion Beach Women Lingerie Set Sleepwears G-string Bra (7668) - EasyShopping24x7 Preços baixos em Banda Hanes Preto Tamanho 38 Sutiãs e conjuntos para mulheres
Preços baixos em Banda Hanes Preto Tamanho 38 Sutiãs e conjuntos para mulheres Summer Two Piece Outfits Girly outfits, Fashion outfits, Pretty outfits
Summer Two Piece Outfits Girly outfits, Fashion outfits, Pretty outfits