In the following compressibility factor (Z) vs. pressure graph 300 K, the compressibility of CH_{4} pressure < 200 bar deviates from ideal behaviour becauseThe molar volume of CH_{4} is than its molar
4.8 (436) In stock

Click here:point_up_2:to get an answer to your question :writing_hand:in the following compressibility factor z vs pressure graph at 300 k the compressibility of
Click here👆to get an answer to your question ✍️ In the following compressibility factor -Z- vs- pressure graph 300 K- the compressibility of CH-4- pressure - 200 bar deviates from ideal behaviour becauseThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is same as that in its ideal stateIntermolecular interactions between CH-4- molecules decreases

Solved The plot below shows how compressibility factor (Z)

Determine Compressibility of Gases

Ideal Gas - an overview

Part 4. Thermodynamics of Gases - W.H. Freeman

4.2: Real Gases (Deviations From Ideal Behavior) - Chemistry LibreTexts

The graph of compressibility factor (Z) :vs: P one mole of a real gas is shown in following diagram. The graph is plotted constant temperature 273 K. If the slope of graph
Real gases
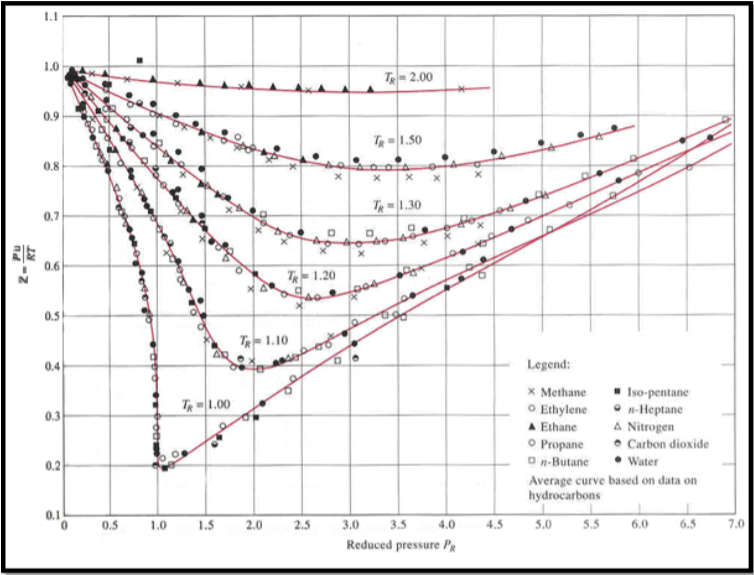
Solved Use the graph of compressibility factors in terms of

Chapter 03 - States of Matter - Module, PDF, Gases

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

Processes, Free Full-Text

Compressibility factor (gases) - Citizendium
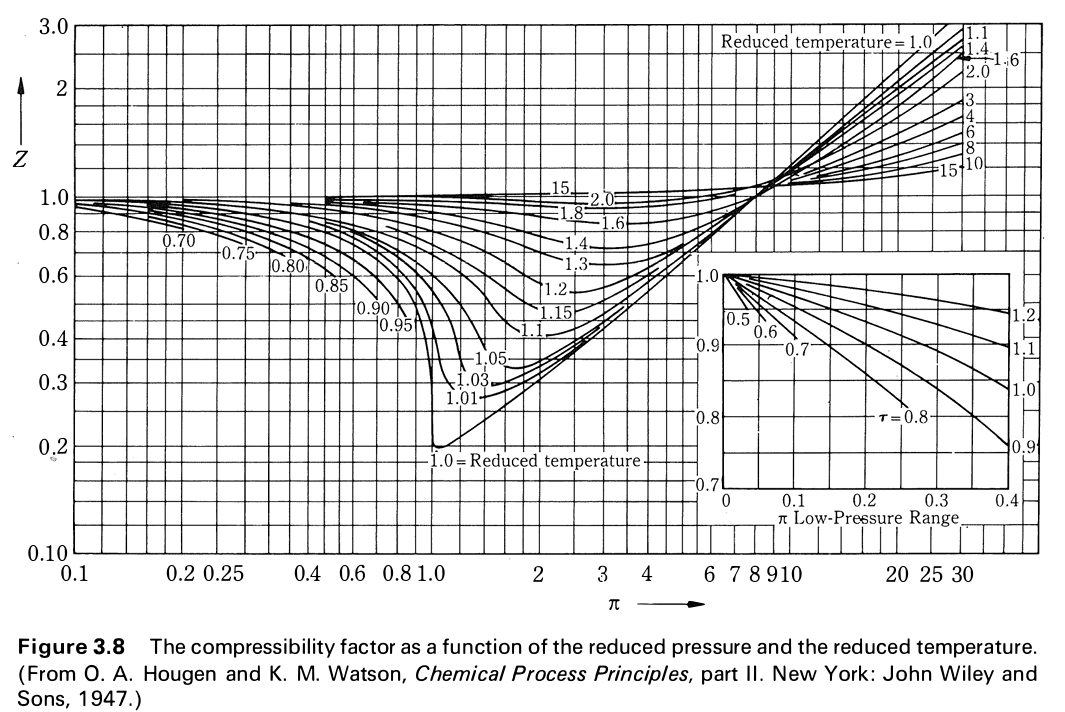
Solved Use the plot of compression factor (Z) vs reduced
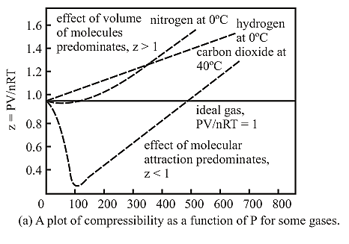
Two flasks A and B have equal volumes A is maintained at 300K and B at 600K While A contain H2 gas B has an equal mass of CH4 gas Assuming ideal
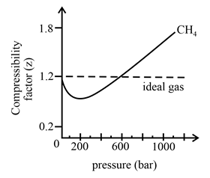
In the following compressibility factor Zvs pressure graph at 300Kthe compressibility of CH4 at pressures 200bardeviates from ideal behaviour because
Standing and Katz gas compressibility factor
Answered: (a)Using the compressibility chart,…
Answer in Molecular Physics Thermodynamics for Neilmar #278440
Calculate the Compressibility Factor 'z' for Hydrocarbon Gases • zFactor
 Result Core Waterproof Overtrousers
Result Core Waterproof Overtrousers Disney sweatpants 2 pack Color lavender - SINSAY - XU532-04X
Disney sweatpants 2 pack Color lavender - SINSAY - XU532-04X- Women's Demi Daydream Push-Up Bra - Auden™ Dark Brown 36B
 Ladies Only Sign Stock Illustration
Ladies Only Sign Stock Illustration 90 Degree By Reflex Women's Side Slit Full Zip Fleece Crop Hoodie
90 Degree By Reflex Women's Side Slit Full Zip Fleece Crop Hoodie Go Cotton Bermuda Shorts [Grey] For Men Online | Hummel India
Go Cotton Bermuda Shorts [Grey] For Men Online | Hummel India
