At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to
4.9 (248) In stock
At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to :
At low pressure- the van der waal-s equation is written as -P- a-V-2-V-RT - Then compressibility factor is then equal to
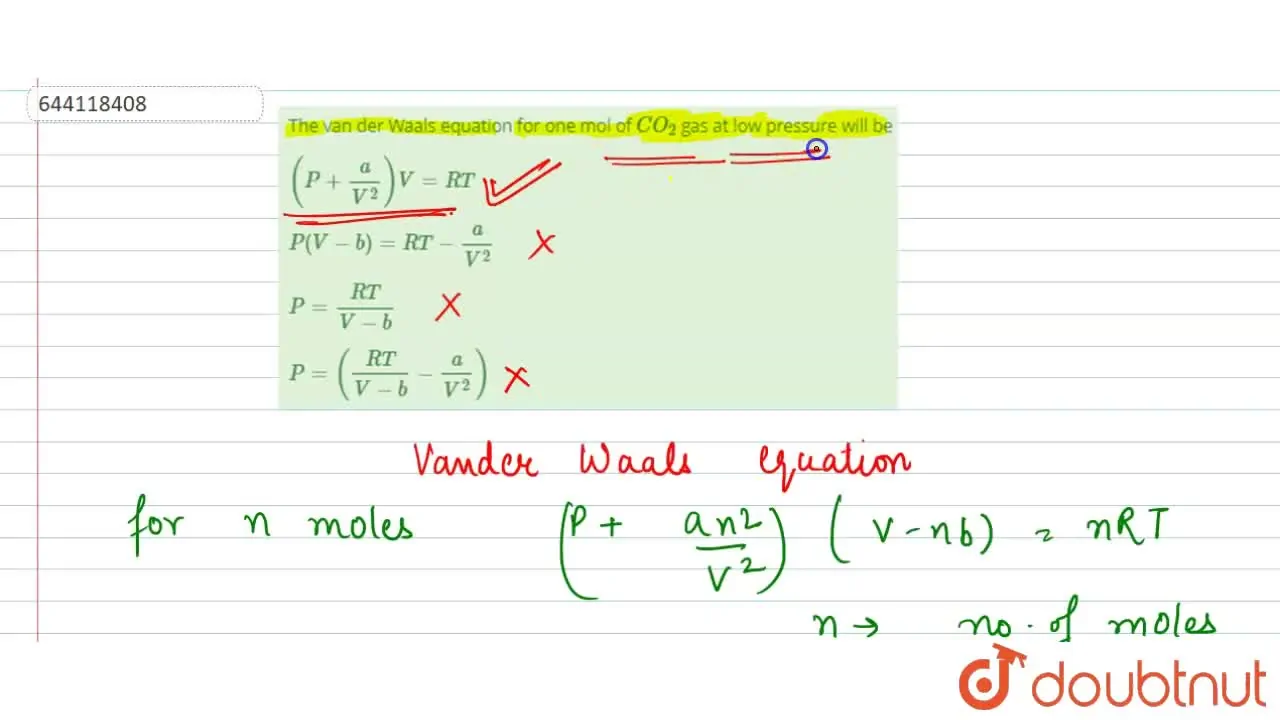
The van der Waals equation for one mol of CO(2) gas at low pressure wi

If Z is a compressibility factor, van der Waals' equation at low press

At low pressure, the compressibility factor is given as (1) RIV RTV RT

Compressibility factor (Z) a real gas moderately low pressure is
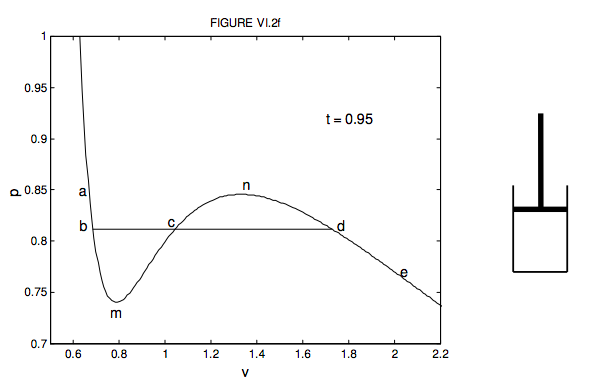
6.3: Van der Waals and Other Gases - Physics LibreTexts

09 DEFINITION Behaviour of gases by van der Waals equation (P+*}(0
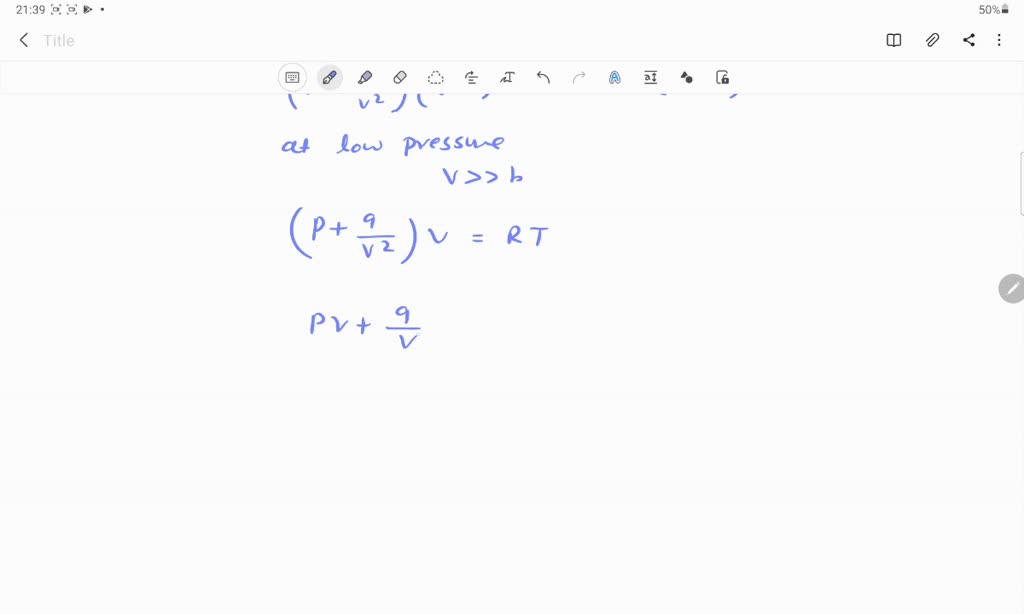
⏩SOLVED:If Z is a compressibility factor, van der Waals equation

Solved 2. (20 points) At low pressures, the compressibility

Van Der Waals Equation - an overview
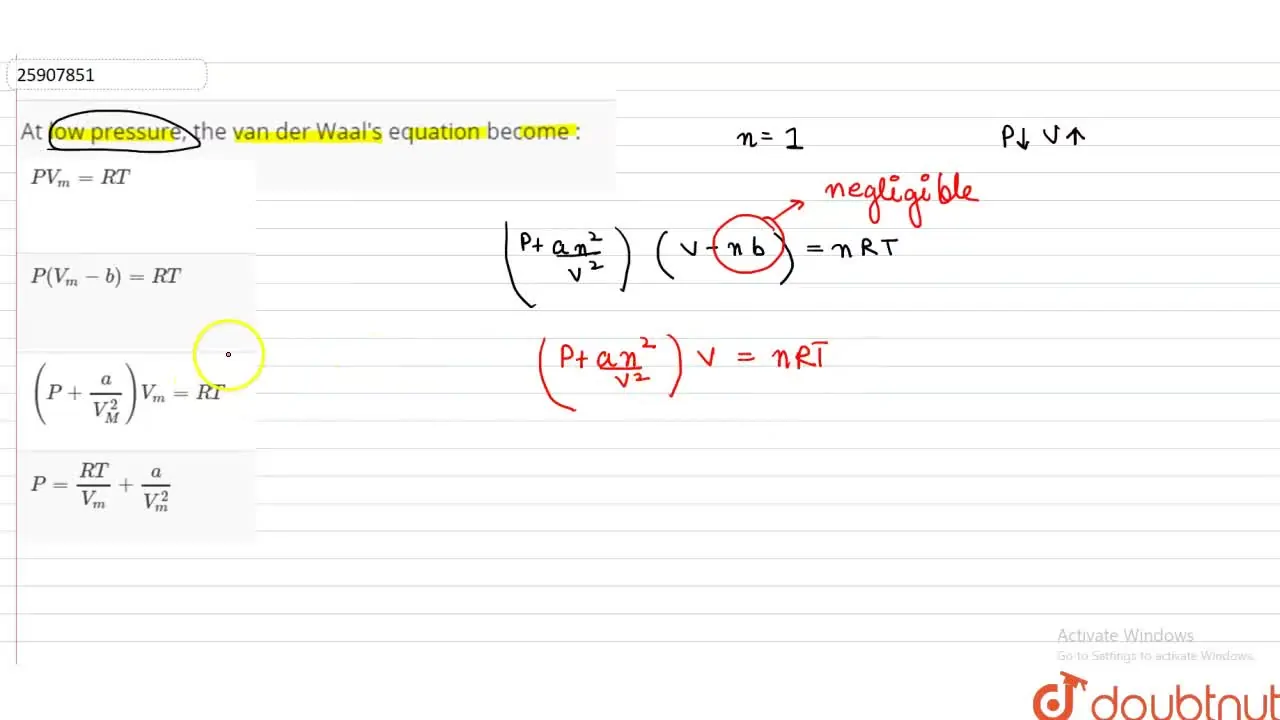
At low pressure, the van der Waal's equation become
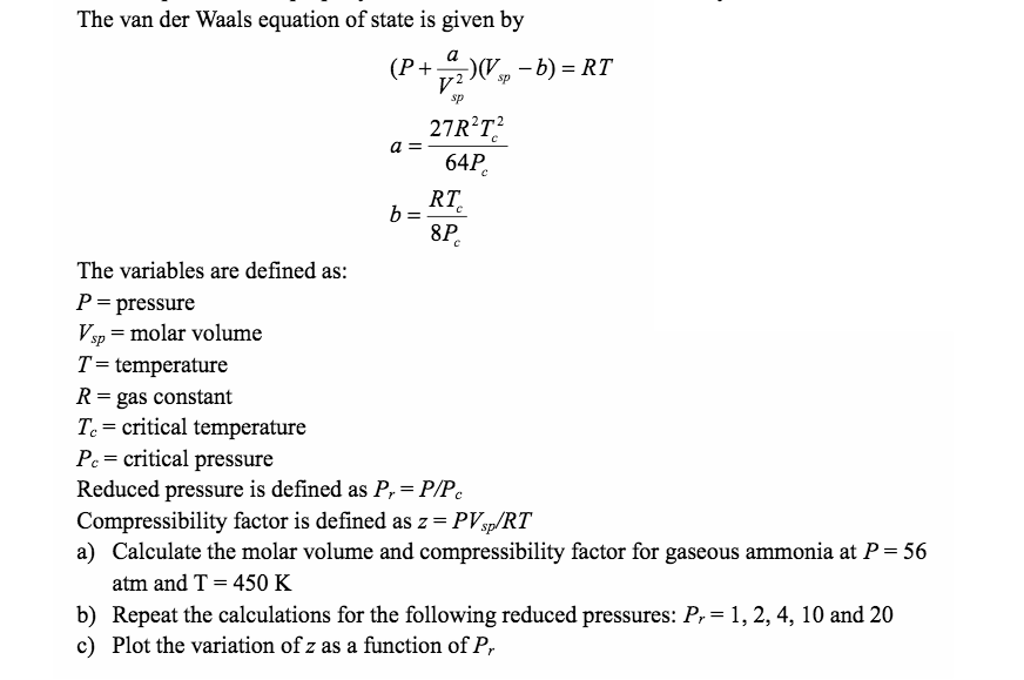
The van der Waals equation of state is given by (P +

At low pressure the van der Waals' equation is reduced to `[P +(a

Van der Waals equation: van der Walls EOS, [Pr*3/Vr^2] [3Vr-1] =
Solved] Why is the compressibility factor less than 1 at most conditions?
Explain how the compression factor varies with pressure and
the equation of state of a gas is p(v-nb)=rt where b and r are consta - askIITians
 Wood Pattern , Marble Pattern Seamless Texture Background Stock Photo, Picture and Royalty Free Image. Image 75042564.
Wood Pattern , Marble Pattern Seamless Texture Background Stock Photo, Picture and Royalty Free Image. Image 75042564. Lover-Beauty Black Thong Bodysuit for Women Tummy Control Deep V Neck body Suit Slim Tank Top at Women's Clothing store
Lover-Beauty Black Thong Bodysuit for Women Tummy Control Deep V Neck body Suit Slim Tank Top at Women's Clothing store China Customized Wet Type EDM Brass Wire Wire Drawing Machine Manufacturers, Suppliers - Factory Direct Price - SSS HARDWARE
China Customized Wet Type EDM Brass Wire Wire Drawing Machine Manufacturers, Suppliers - Factory Direct Price - SSS HARDWARE BNWT Lululemon Wunder Under Leggings, Women's Fashion, Activewear on Carousell
BNWT Lululemon Wunder Under Leggings, Women's Fashion, Activewear on Carousell STYLE ENCORE CHESAPEAKE - 11 Photos & 10 Reviews - 1437 Sams Dr, Chesapeake, Virginia - Women's Clothing - Phone Number - Yelp
STYLE ENCORE CHESAPEAKE - 11 Photos & 10 Reviews - 1437 Sams Dr, Chesapeake, Virginia - Women's Clothing - Phone Number - Yelp- Women's Simply Vera Vera Wang Printed Tie-Dye Live-In Leggings
