SOLVED: Problem 3 (35 marks): Calculate the compressibility factor Z and specific volume V cm/mole for ethane at 47.5°C and 25 bar by the following equations: 1. Ideal gas equation - 5
5 (560) In stock
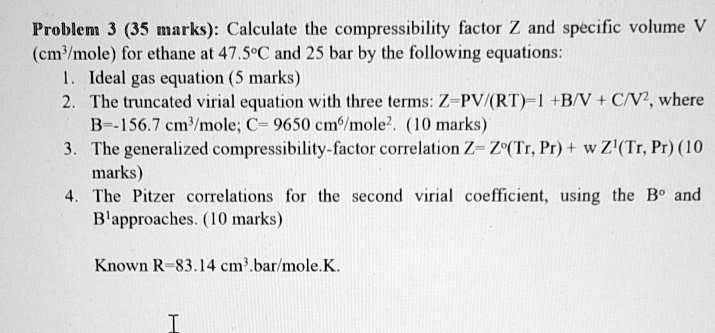

SOLVED: Question 3 (35 points): Calculate the molar volume and the

SOLVED: Problem 3 (35 marks): Calculate the compressibility factor
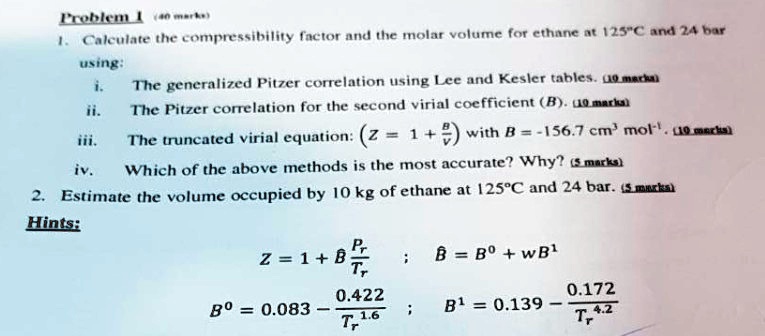
SOLVED: Problem 1: Calculate the compressibility factor and the

SOLVED: Question 3 (35 points): Calculate the molar volume and the
SOLVED: Problem 3 (35 marks): Calculate the compressibility factor

SOLVED: Problem 3 (35 marks): Calculate the compressibility factor
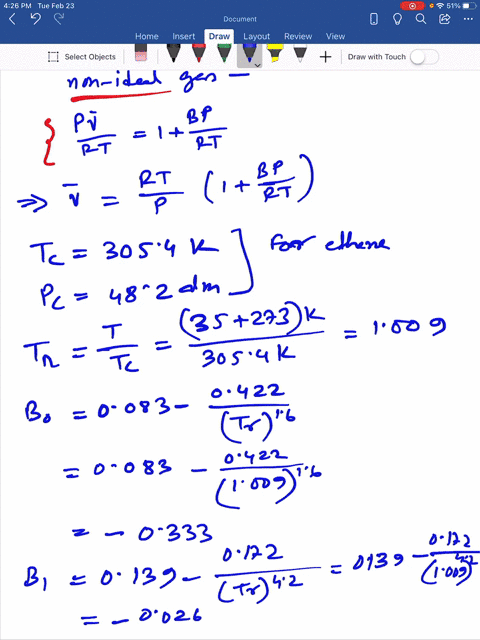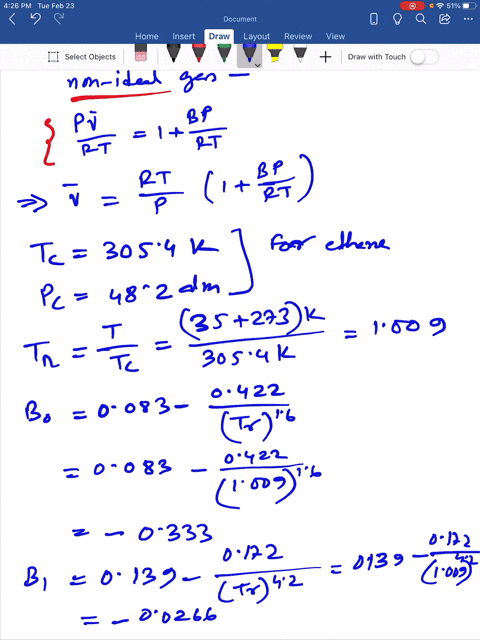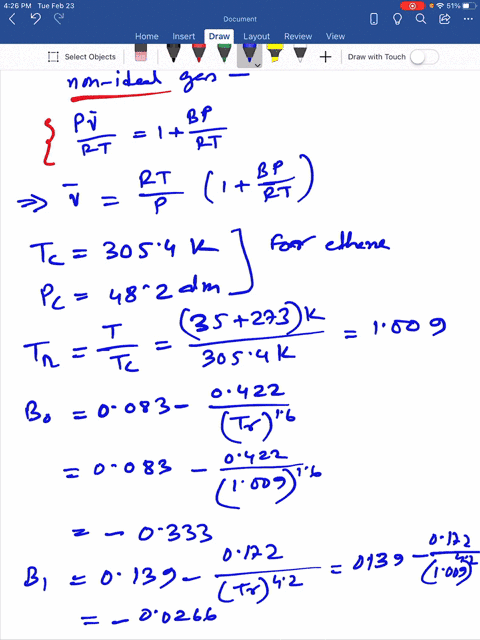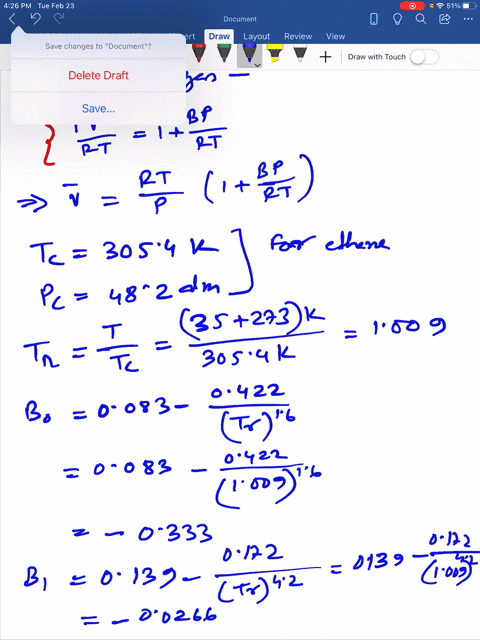
SOLVED: Question 3 (35 points): Calculate the molar volume and the

SOLVED: Problem 3 (35 marks): Calculate the compressibility factor

SOLVED: 25 points) Calculate the volume of 1 mole of Ethane at
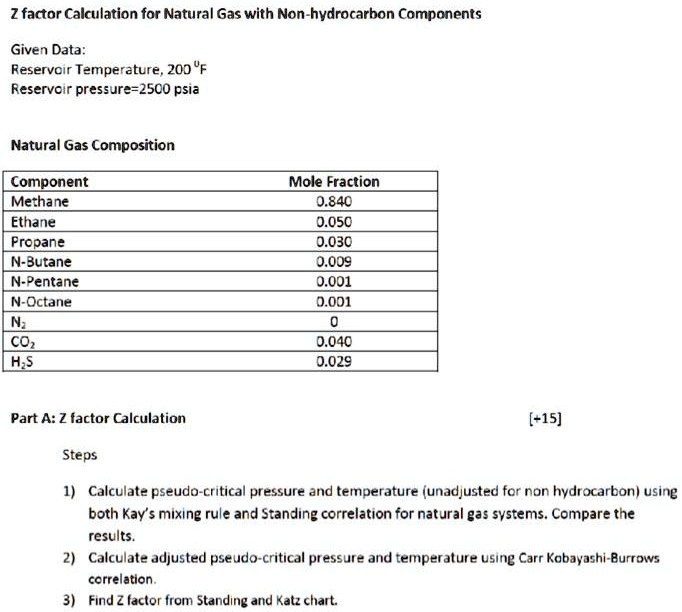
SOLVED: 3) Find Z factor from Standing and Katz chart. Z factor
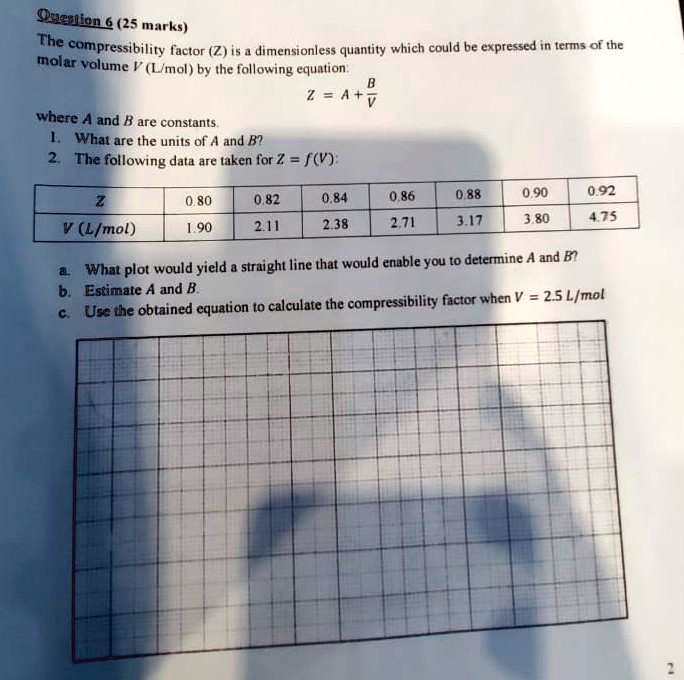
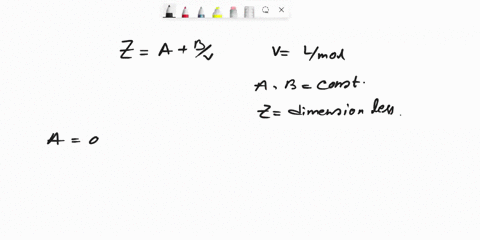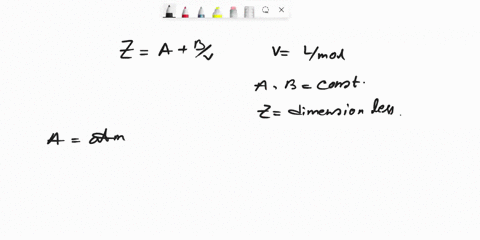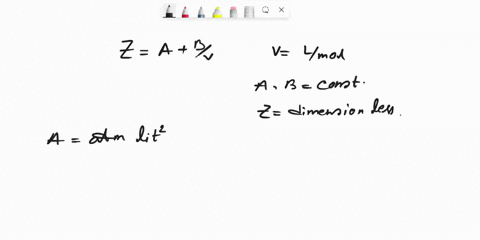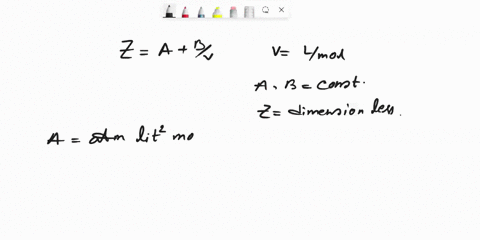
SOLVED: Question 6 (25 marks) Given Z = A + B, where A and B are
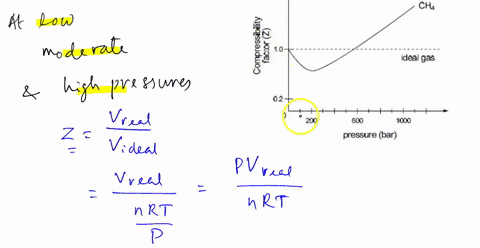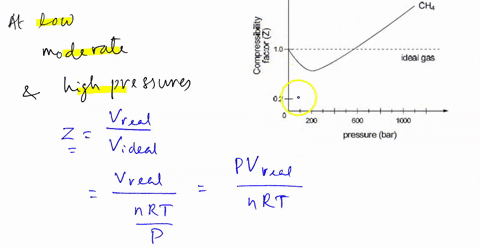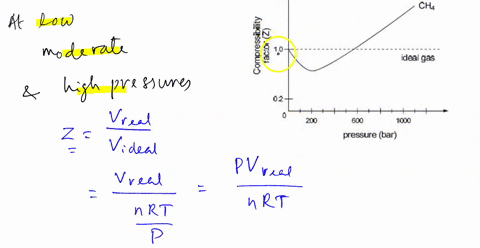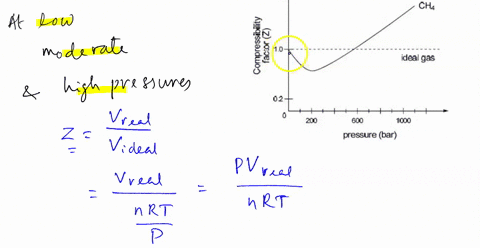
SOLVED: 3) Find Z factor from Standing and Katz chart. Z factor
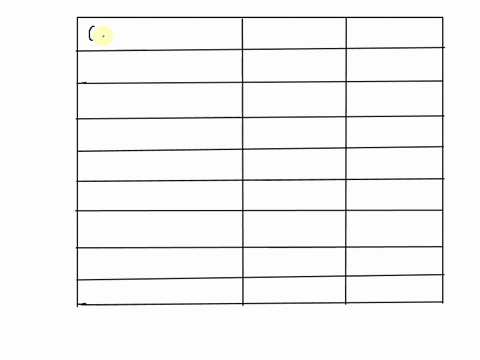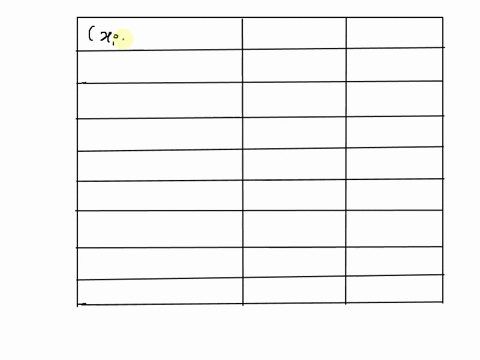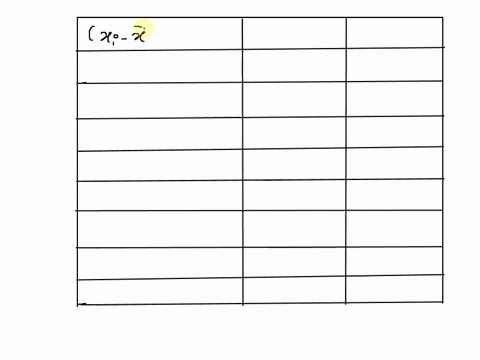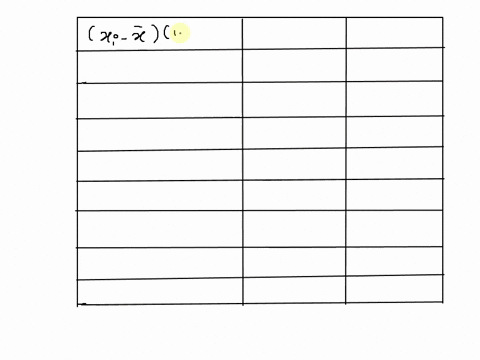
SOLVED: 25 points) Calculate the volume of 1 mole of Ethane at
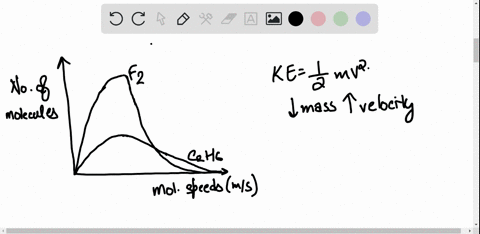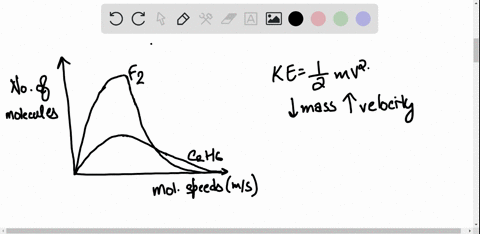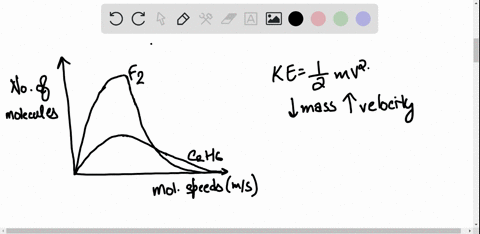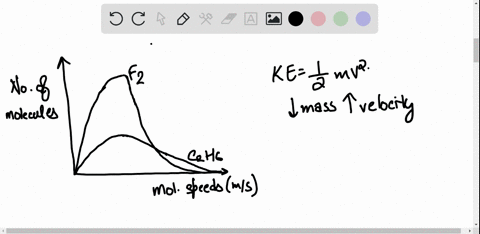
SOLVED: 3) Find Z factor from Standing and Katz chart. Z factor
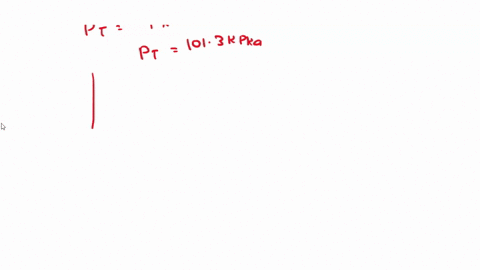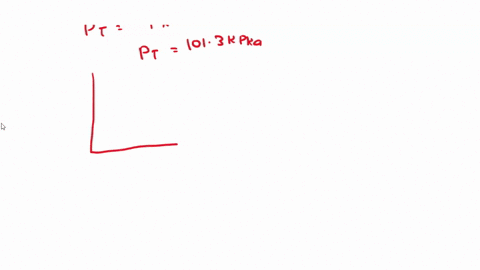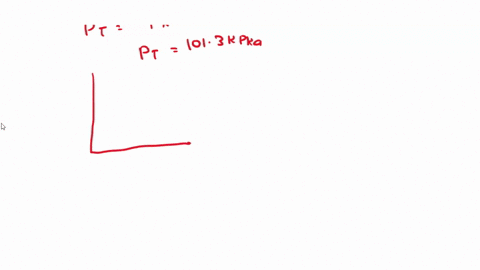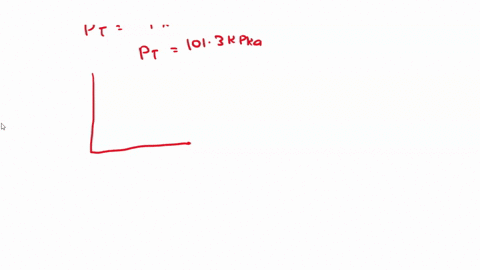
SOLVED: 3) Find Z factor from Standing and Katz chart. Z factor
What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0
3.2 Real gas and compressibility factor – Introduction to
Real gasses For an ideal gas, the compressibility factor Z = PV
Slope of graph of compressibility factor(Z) with pressure(P) for
 THE WORLD OF SPAGHETTI FASHION – Street Style Stalk
THE WORLD OF SPAGHETTI FASHION – Street Style Stalk A date with Hu Tao comic [Ch. 1-5] by @pic_postcard [Translated] Genshin Impact
A date with Hu Tao comic [Ch. 1-5] by @pic_postcard [Translated] Genshin Impact Lace Draped Corset Skater Dress White - Luxe Midi Dresses and Celebrity Inspired Dresses
Lace Draped Corset Skater Dress White - Luxe Midi Dresses and Celebrity Inspired Dresses FINETOO 6 Pack Women's Seamless Hipster Underwear Togo
FINETOO 6 Pack Women's Seamless Hipster Underwear Togo Straight Leg Moss Green Nylon Cargo Pants – JWS
Straight Leg Moss Green Nylon Cargo Pants – JWS Barnwood Concealed Sofa Table
Barnwood Concealed Sofa Table