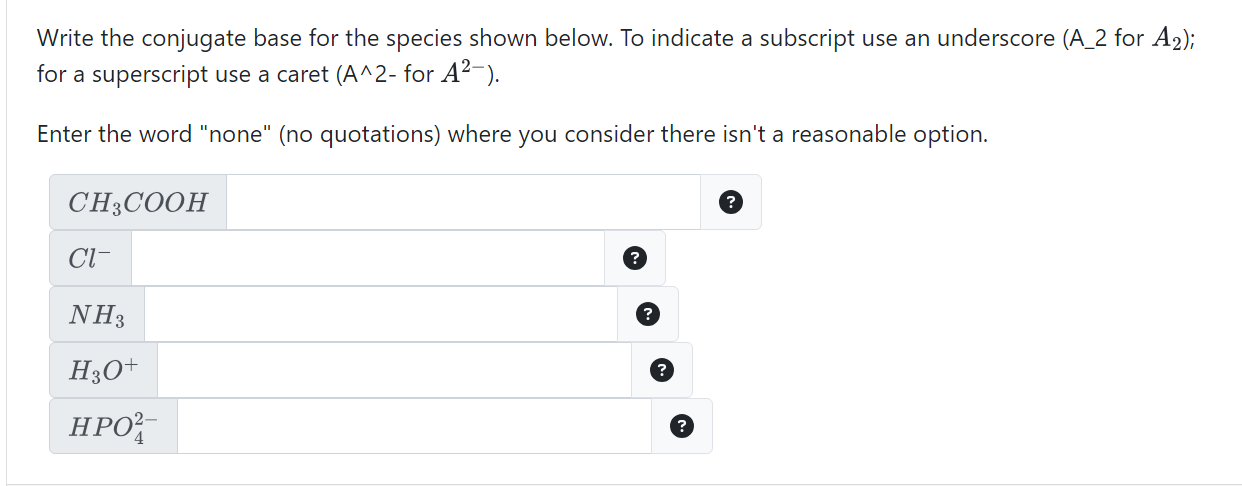
Solved Write the conjugate base for the species shown below
4.8 (761) In stock

For the chemical equations shown below, label each reactant as either acid or base, and each product as either conjugate acid or conjugate base according to the Bronsted-Lowry definition. [{Image src
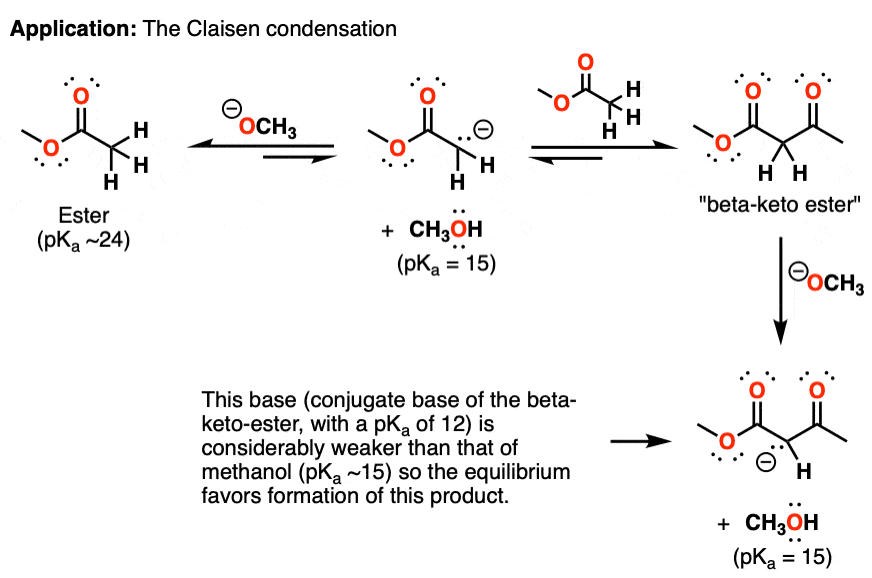
Reversible and Irreversible Acid-Base Reactions In Organic Chemistry

For each conjugate acid-base pair, identify the first species as an acid or a base and the second species as its conjugate acid or base. In addition, draw Lewis structures for each
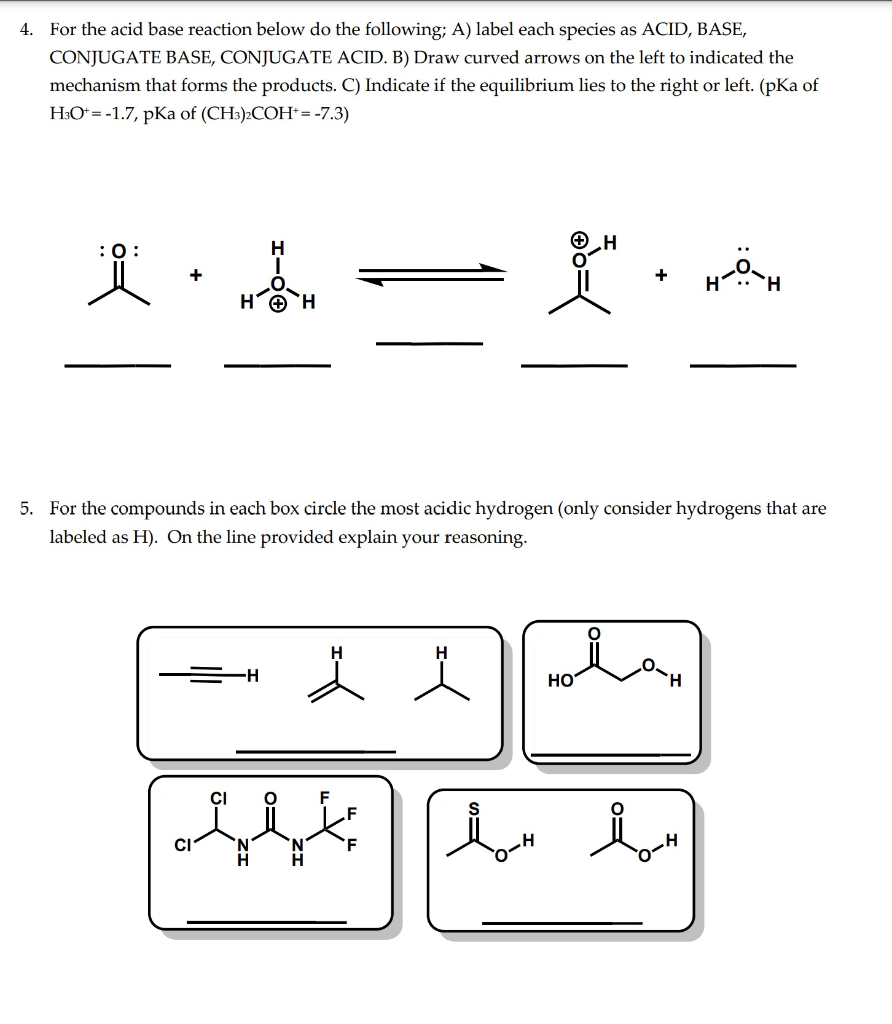
Solved 4. For the acid base reaction below do the following;

For the following reaction, identify the reactant that is an acid, the reactant that is a base, and the two conjugate acid-base pairs present. OH-(aq) + HNO2(aq) arrow H2O(l) + NO2-(aq)
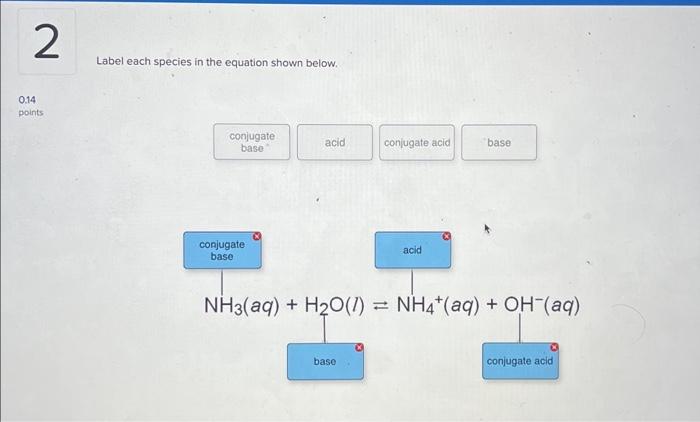
Solved Label each species in the equation shown

How to Choose an Acid or a Base to Protonate or Deprotonate a Given Compound - Chemistry Steps
Solved) - (Part of post lab questions 1, 2, and 9) I need help drawing all (1 Answer)

In the Acid-Base reaction shown below, write the structure of the conjugate base of tropolone. Using curved arrow notation write down all possible resonance structures for this conjugate base.
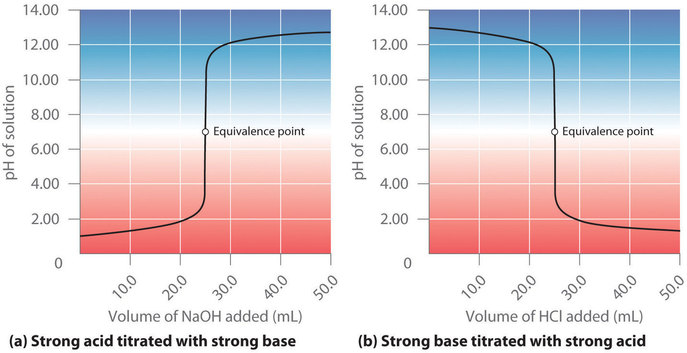
15.6: Acid-Base Titration Curves - Chemistry LibreTexts

The following diagrams represent aqueous solutions of three acids
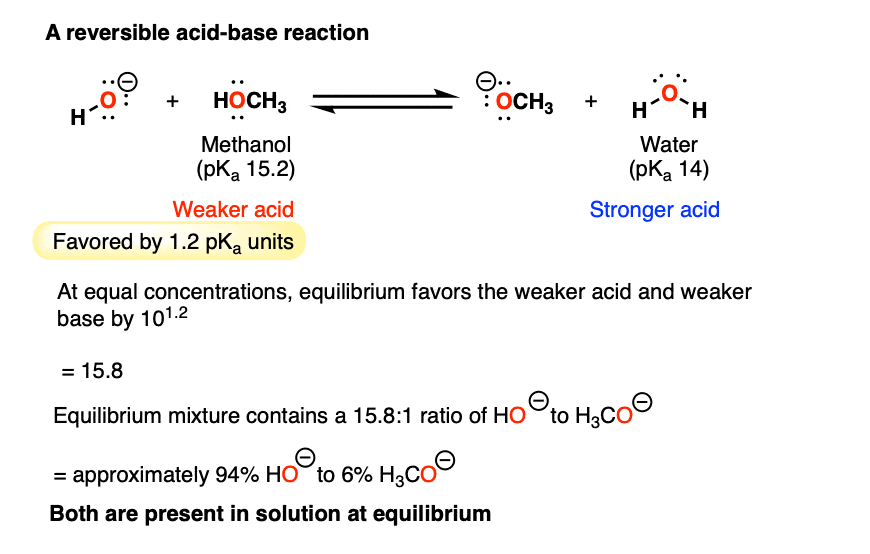
Reversible and Irreversible Acid-Base Reactions In Organic Chemistry
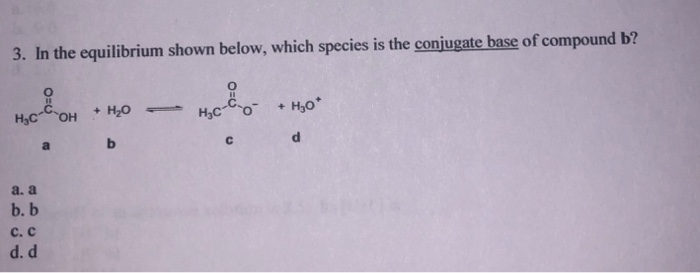
Solved 3. In the equilibrium shown below, which species is
Solved] Please help me answer questions below Write the formula of the
Page 6 - Milliken Product Portfolio 2018-2019
Feminine character base by vicnick-underscore on DeviantArt
Kohler - Underscore Rectangle 60 X 32 Drop-In Bath - White
javascript - underscore__WEBPACK_IMPORTED_MODULE_3__ is not a
Create Custom WordPress Themes with Underscores Components - Hongkiat
 How To Style Bob Braids
How To Style Bob Braids/product/79/9828142/1.jpg?1541) Generic Women Solid Cargo Pants Multicolor Stretch Casual Lacing Drawstring High Waist Bottoms Trousers Fitness Tracksuit Dropshipping(#Black)
Generic Women Solid Cargo Pants Multicolor Stretch Casual Lacing Drawstring High Waist Bottoms Trousers Fitness Tracksuit Dropshipping(#Black) Wimbledon Logo-Embroidered Appliquéd Cotton-Blend Cardigan
Wimbledon Logo-Embroidered Appliquéd Cotton-Blend Cardigan Cork Reversible Belt, Vegan Belt for Men Made in Portugal
Cork Reversible Belt, Vegan Belt for Men Made in Portugal Bra-La Bra Strap 3 colors — Distinctively Hers
Bra-La Bra Strap 3 colors — Distinctively Hers Duluth Trading Co Pants Womens 12x29 Black Ponte Knit Stretch WearWithAll Size 12 - $25 - From Laura
Duluth Trading Co Pants Womens 12x29 Black Ponte Knit Stretch WearWithAll Size 12 - $25 - From Laura