The compression factor (Z) Co, 7°C and 100 atm is 0.21. Calculate the volume of a 4 mole sample of co, same temperature and pressure (use R = 0.08 L. atm/K.mol (1)
4.9 (392) In stock

Click here:point_up_2:to get an answer to your question :writing_hand:the compression factor z for co at 7c and 100atm is 021 calculate the volume
Click here👆to get an answer to your question ✍️ The compression factor -Z- Co- 7-C and 100 atm is 0-21- Calculate the volume of a 4 mole sample of co- same temperature and pressure -use R - 0-08 L- atm-K-mol -1- 0-192 -2- 0-05 L -3- 0-38 L -4- 0-44 L closed container can be

Solucionario Felder, Química y ciencias

The compression factor compressibility factor for 1 mole of a van der Waals' gas at 0∘ C and 100 atmospheric pressure is found to be 0.5 . Assuming that the volume of

Solved (Triple-Play Bonus) For a certain gas, the

Compression Factor Exam Problem using Molar Volumes - Fully Explained!
Is there a set of conditions at which the compression factor
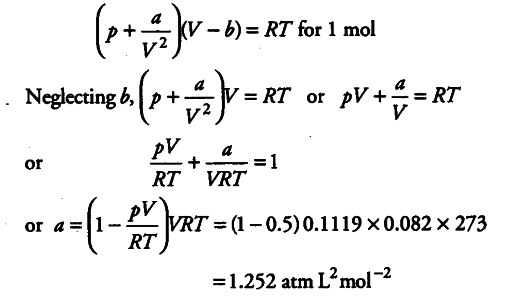
The compression factor (compressibility factor) for one mole of a van - CBSE Class 11 Chemistry - Learn CBSE Forum

Solved A sample of 4.0 molO2( g) is originally confined in

Gaskell Laughlin Solutions, PDF, Heat

Modeling Trasport phenomena Part 1 by Alireza Rezayee - Issuu

29. (4) 80% The compression factor (Z) Co, 7°C and 100 atm is 0.21. Calculate the volume of a 4 mole sample of Co, same temperature and pressure (use R = 0.08

Compression Factor Exam Problem using Molar Volumes - Fully Explained!
Atmosphere, Free Full-Text

Acentric Factor - an overview

The compression factor (Z) for CO2 at 7∘C and 100 atm is 0.21. Calculate..
Real gas z-factor, as attributed to Standing and Katz, 9 plotted as a
A To Z Liquid (Botnical Growth Factor), Bottle, Packaging Size: 1 Litre at Rs 963/litre in Jalgaon
Real-gas z-factor, as attributed to Standing and Katz, 9 plotted
 Nike, Pants & Jumpsuits, Nike Sweat Pants Joggers Grey Womens Size Small
Nike, Pants & Jumpsuits, Nike Sweat Pants Joggers Grey Womens Size Small 4029 Leah Seamless Swirl Jacquard Microfiber Softcup – Bahamas
4029 Leah Seamless Swirl Jacquard Microfiber Softcup – Bahamas Lovely Vintage Portrait Photo Photograph Celluloid Button Lovely Lady Portrait
Lovely Vintage Portrait Photo Photograph Celluloid Button Lovely Lady Portrait Spell & The Gypsy Collective - Folk Song Boho Dress on Designer
Spell & The Gypsy Collective - Folk Song Boho Dress on Designer The truth about your smelly armpits — how to tackle body odour
The truth about your smelly armpits — how to tackle body odour Silicone Sticky Bra Adhesive Invisible Bra Backless
Silicone Sticky Bra Adhesive Invisible Bra Backless