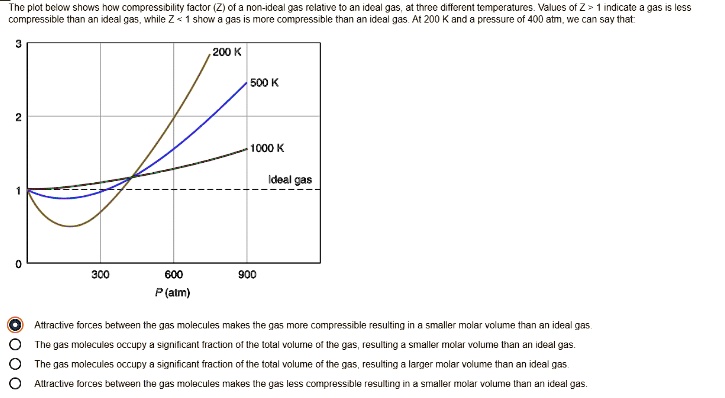
SOLVED: Plot bclon shcs now compressibility factor (Ziofa non-Idc? 935 relative to an ideal gas; J1 force differential Mocraiurc: Values of Z indicate compressibility and inan re any more compressible ideal gas
4.5 (688) In stock
VIDEO ANSWER: We are going to see the difference between the liquid and the guest. The chemical entities are associated with bonds in gas. There are chemical entities away from each other. The guests here have a random motion system that is
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.

What is the value of compressibility factor for a non-ideal gas? - Quora

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

Compressibility factor (z): real gases deviate from ideal behav-Turito
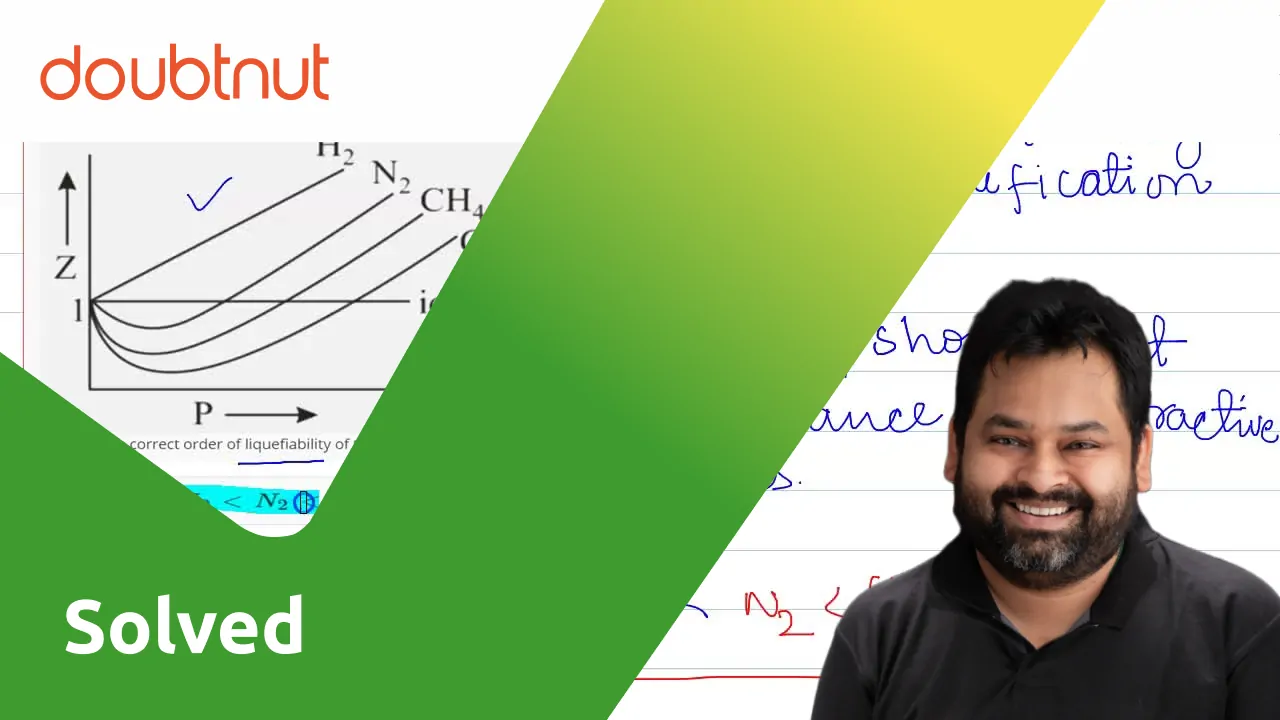
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure
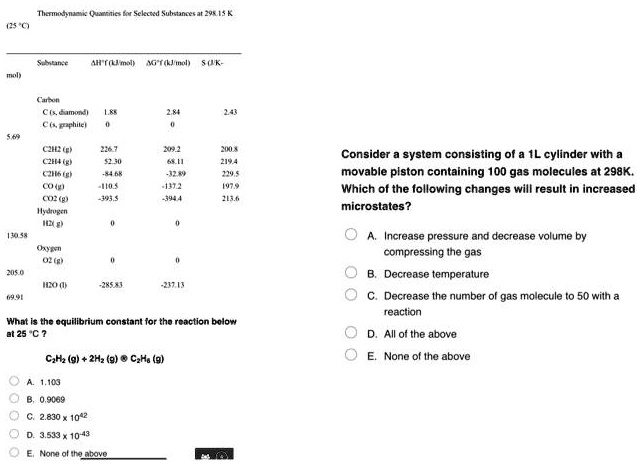
SOLVED: Maena ieli 4 HSEIAL A mall SuK- CEon CamonJi C Fchllcl Qib( CZIHUF Cih Cui CO Consider a system consisting of a cylinder with a movable piston containing 100 gas molecules

The role of the compressibility factor Z in describing the volumetric behavior of gases

Energies, Free Full-Text
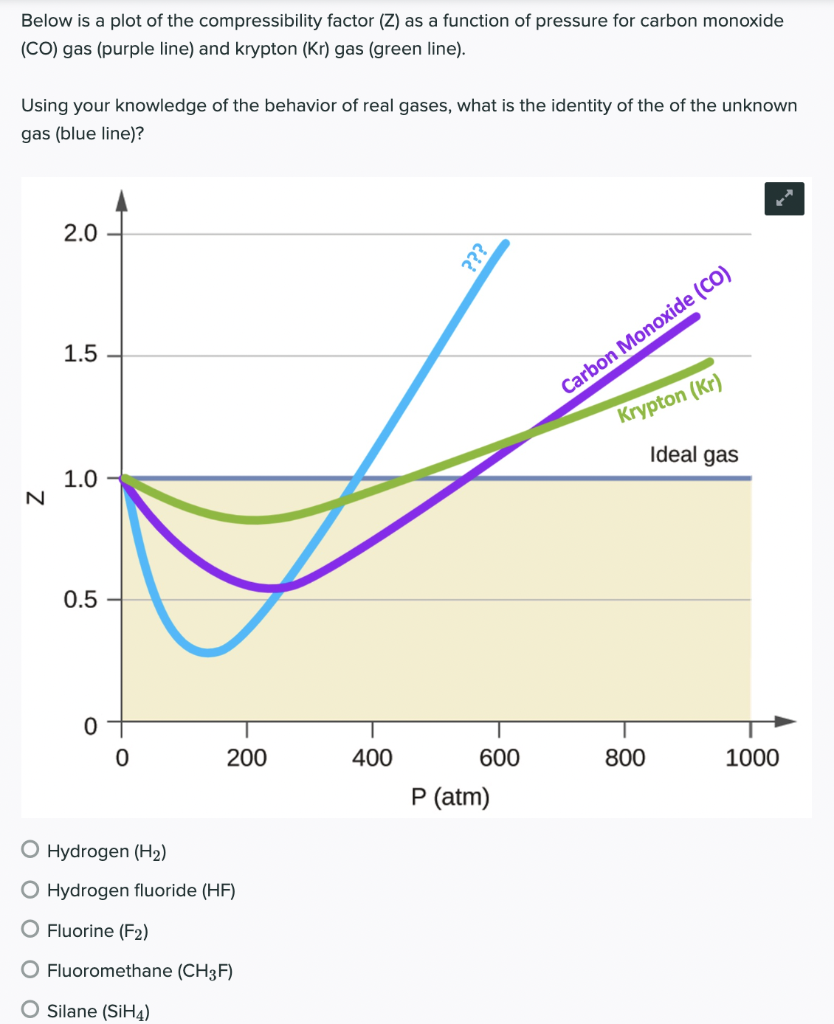
Solved Below is a plot of the compressibility factor (Z) as

Gas Z Factor Calculator: Dranchuk-Abou-Kassem · PVT Solver

gas laws - Compressible Factor - Chemistry Stack Exchange

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange
Compressibility factor Z as function of temperature T with lines
 Buy Navy Blue Pants for Women by De Moza Online
Buy Navy Blue Pants for Women by De Moza Online Yahoo Plus brings you more of what you love while protecting your life online
Yahoo Plus brings you more of what you love while protecting your life online- Classificação de pares de ângulos - Matemática : Explicação e
 Merino & More Women's Functional Base Layer Wool 3/4 Length - S
Merino & More Women's Functional Base Layer Wool 3/4 Length - S long tall sally, Pants & Jumpsuits, Lts Long Tall Sally Leatherette Faux Leather Leggings Pants Nwt
long tall sally, Pants & Jumpsuits, Lts Long Tall Sally Leatherette Faux Leather Leggings Pants Nwt Leggings Women Gym Scrunch Bum Women's Ribbed Leggings Push
Leggings Women Gym Scrunch Bum Women's Ribbed Leggings Push