At a given temperature T gases Ne Ar Xe and Kr are found to deviate from ideal gas behavior (JEE MAINS 2019) - Doctor Logics Sunny Garg Chemistry
5 (253) In stock

At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behavior. Their equation of state is given as P=RTV−b at T. Here, b is the van der Waals constant. Which gas will exhibit steepest increase in the plot of Z (compression factor) vs P?

In the given figure an ideal gas changes its state from `A` to state `C` by two paths `ABC` and

PDF) Thermal energy storage Diego Armando Gutierrez Diaz

Chaudhery Mustansar Hussain - Handbook of Environmental Materials Management-Springer International Publishing (2019), PDF, Environmental Remediation

PDF) Thermal energy storage Diego Armando Gutierrez Diaz

At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behaviour. Their equation of state is given as p = dfrac {RT}{V - b}

At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behavior.

PDF) Ratnakant Paper II jai sankar gummapu
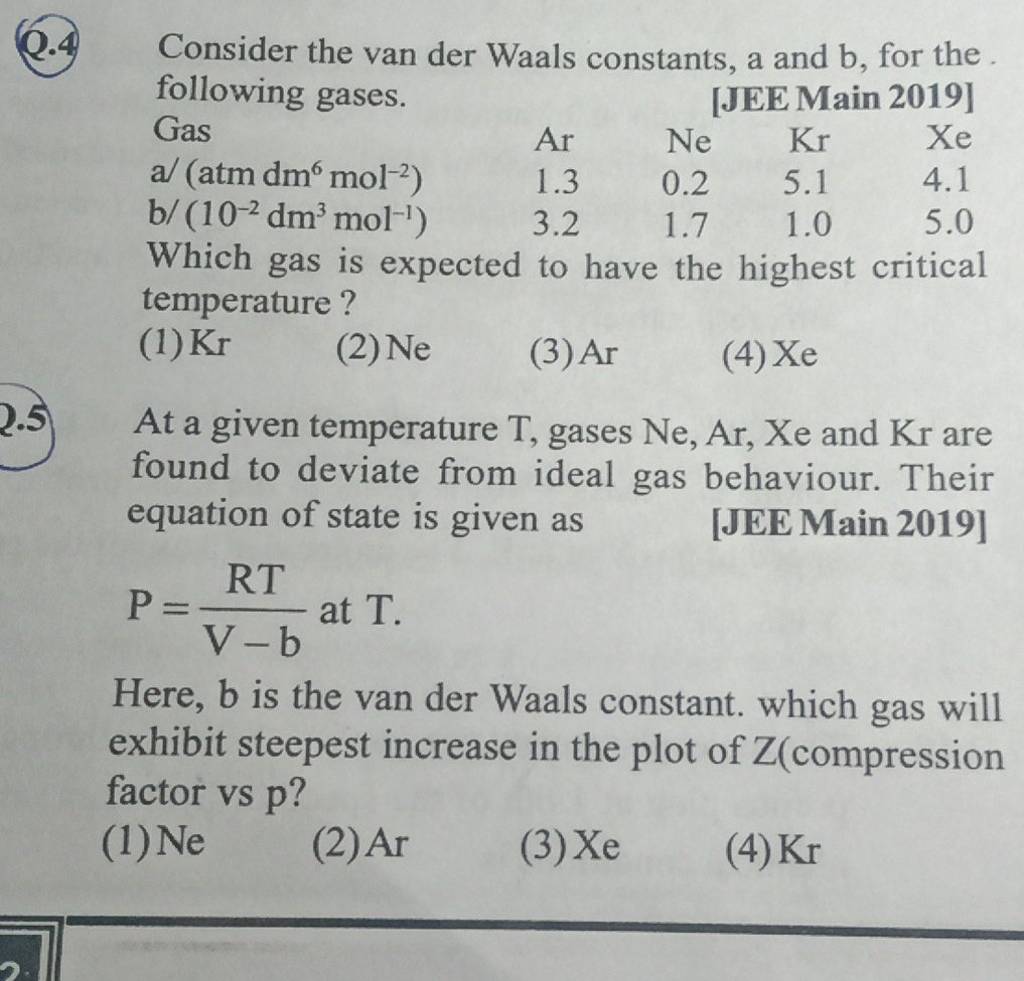
At a given temperature T, gases Ne,Ar,Xe and Kr are found to deviate from..

Solved In general, real gases behave most ideally at high

At a given temperature T, gases Ne,Ar,Xe and Kr are found to deviate from..

Modern Techniques in Biosensors Detection Methods and Commercial Aspects (Gorachand Dutta, Arindam Biswas Etc.), PDF, Biosensor

Solved 4.11 Nenideal Gias Feuatien! axi. where R is the

Ideal gas law assignment 1 - CHEM 1050 Ideal Gas Law Problems Name: An - Studocu

Competition Science Vision - February 2008, PDF
Kinetic Theory of Gases - JEE Main Previous Year Questions with Solutions
Solved The compression factor for a gas is 0.79 at 300 K and
Solved 1. The compression factor, Z of a gas is 0.625. Which
Write an equation for the transformation of y=x vertical compression by a factor of 1/11
53 pts!! The function f(x)= 7^x+1 is transformed to function g
 Jelenew Cycling Bodysuit For Women
Jelenew Cycling Bodysuit For Women DC Life x Probus NYC Admiral Sport “Probot”
DC Life x Probus NYC Admiral Sport “Probot” Vintage 80s Wrestling Sensational Sherri T shirt art !!!! Leggings for Sale by Mikeyofthe80s
Vintage 80s Wrestling Sensational Sherri T shirt art !!!! Leggings for Sale by Mikeyofthe80s Civil War Marine Belt Buckle with Eagle and Anchor - Antique Vintage Style
Civil War Marine Belt Buckle with Eagle and Anchor - Antique Vintage Style 9685 Sculptresse by Panache Estel Full Cup Bra - 9685 Lagoon
9685 Sculptresse by Panache Estel Full Cup Bra - 9685 Lagoon Truekind® Enhanced Comfort Wireless Shaper Bra Size Med/Black + Bra Extender
Truekind® Enhanced Comfort Wireless Shaper Bra Size Med/Black + Bra Extender