Ideal Gas Assumptions - Kinetic Theory
4.8 (616) In stock

When considering a gas as an ideal gas and applying the ideal gas law pV=nRT, we need to make 4 assumptions. (1) The volume of a molecule within the gas is n
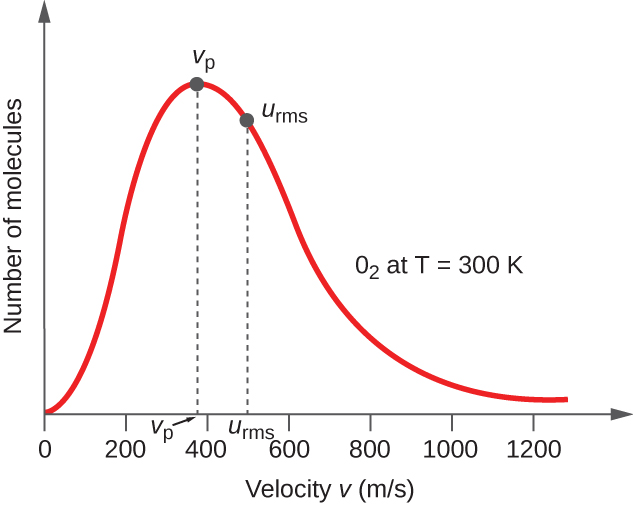
5.4 The Kinetic-Molecular Theory – Chemistry

1-4 Gases (Part 3)

Thermodynamics. The Ideal Gas Law Assumptions The particles of a
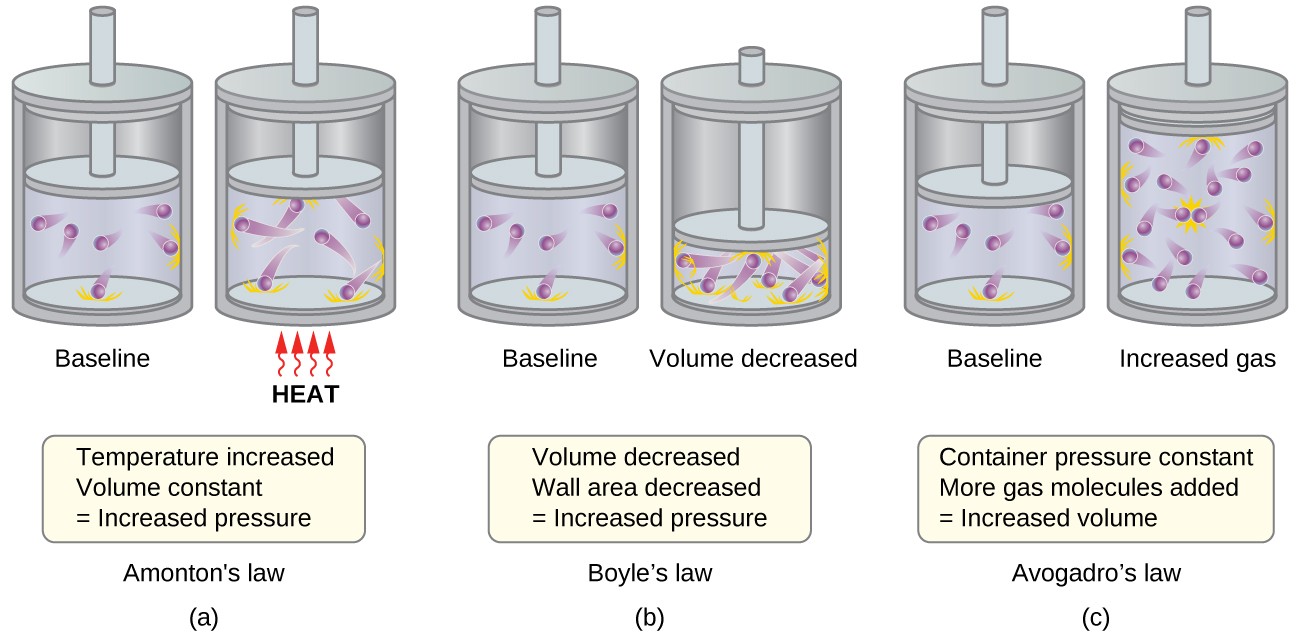
The Kinetic-Molecular Theory
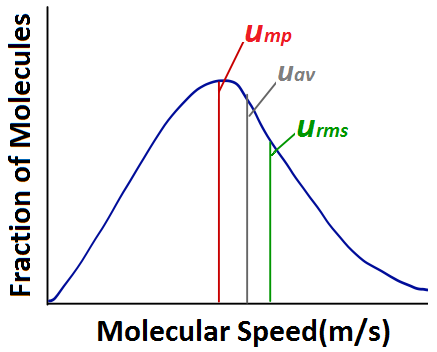
Kinetic Molecular Theory of Gases – Introductory Chemistry – 1st

Physics 32 Kinetic Theory of a Gas (10 of 10) Time Between Collision
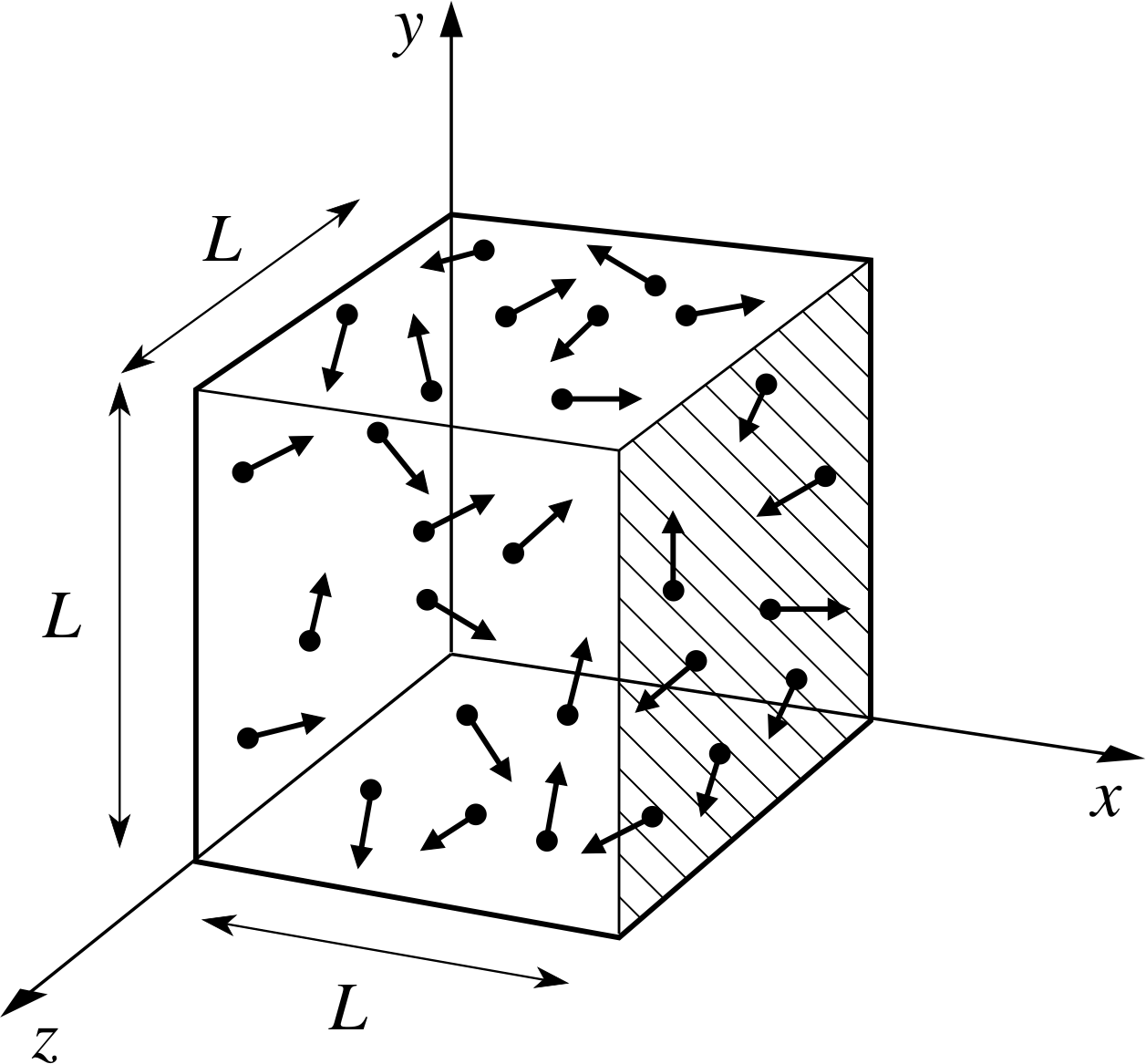
PPLATO, FLAP

Solved 11 Which of the following assumptions is NOT * ?made
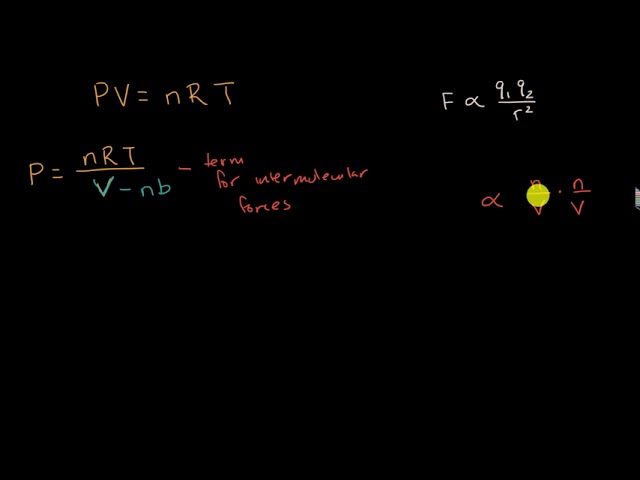
Assumptions in Ideal Gas Model - IB Physics Chapter 3.2 (Part 3)
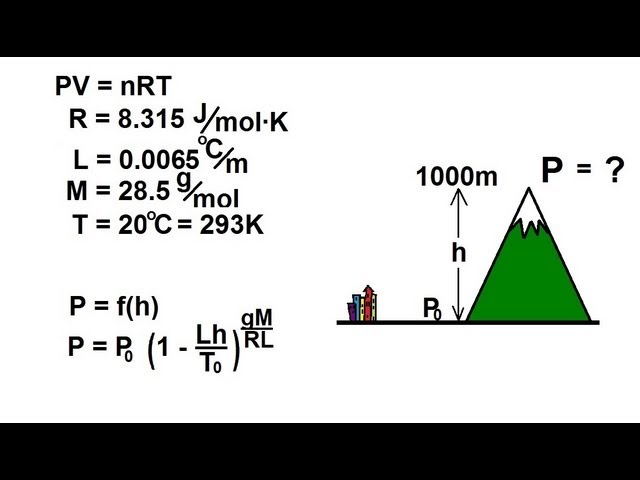
Physics 32 Kinetic Theory of a Gas (10 of 10) Time Between Collision
365 Days of Climate Awareness 33 — The Ideal Gas Law, by The Good Men Project, Greener Together
Ideal Gases - IB Physics Stuff
PPT - The Ideal Gas Law 01 PowerPoint Presentation, free download - ID:3194926
6.5.3 Ideal Gas Equation, AQA A Level Physics Revision Notes 2017
 Shapermint Compression Wirefree High Support Bra
Shapermint Compression Wirefree High Support Bra- Pacific Ocean big wave surfing – 20 Foot Plus video
 Goddess Alice GD6040 Nude Wire Free Soft Cup Bra
Goddess Alice GD6040 Nude Wire Free Soft Cup Bra Chic 2020 Hi Low Tulle Skirt With Ruffled Detail, Strapless Sheer
Chic 2020 Hi Low Tulle Skirt With Ruffled Detail, Strapless Sheer Women Seamless Bandeau Bra Tube Top Plus Size XL 1X 2X 3X 4X No
Women Seamless Bandeau Bra Tube Top Plus Size XL 1X 2X 3X 4X No Cathalem Smoothing Comfort Underwire Lightly Lined T-Shirt Bra Bras for Women Comfort(Beige,M)
Cathalem Smoothing Comfort Underwire Lightly Lined T-Shirt Bra Bras for Women Comfort(Beige,M)
