Draft Guidance Document: Applications for Medical Device Investigational Testing Authorizations
4.5 (222) In stock

This draft guidance document reflects Health Canada’s current thinking on Investigational Testing Authorizations (ITA) for medical devices and may be subject to changes as policy develops. The document clarifies application requirements and processes, including pre-ITA meetings, format for an ITA application and filing requests for revisions to an ITA.

Complex software algorithms are the real reason FDA is after Laboratory Developed Tests, by Bethany Hills Grois, HLWF ™ Alliance

Guidance on how to complete the application for a new medical device licence: Overview

Regulatory guidelines and preclinical tools to study the biodistribution of RNA therapeutics - ScienceDirect
The FDA Regulatory Landscape For AI In Medical Devices

Current Medical Device Regulations in Canada

Canada's Health Canada - Global Regulatory Partners, Inc.

Guidance Document: Pre-market Requirements for Medical Device Cybersecurity
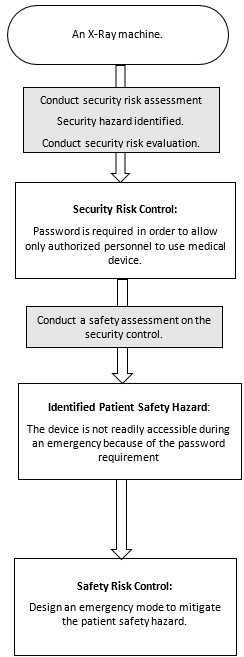
Guidance Document: Pre-market Requirements for Medical Device Cybersecurity

Applications for Medical Device Investigational Testing Authorizations Guidance Document
%20A%20Complete%20Guide%20to%20Bringing%20a%20Medical%20Device%20to%20Market.png?width=4250&name=(cover)%20A%20Complete%20Guide%20to%20Bringing%20a%20Medical%20Device%20to%20Market.png)
The Difference Between Intended Use and Indications of Use (And Why These Statements Are So Important)

FDA 2022 annual report shows steady rate of medical device submission reviews
ITA-MED TLSO-250 Complete Posture Corrector Back Support Brace for Women Size M
ITA-MED Breathable Elastic Rib Brace, Best Rib Belt for Women
 Maternity Nursing Sleep Bra Cross Wrap Breastfeeding No Underwire Padded Soft Back Smoothing Bra Pregnancy Breastfeeding Bra
Maternity Nursing Sleep Bra Cross Wrap Breastfeeding No Underwire Padded Soft Back Smoothing Bra Pregnancy Breastfeeding Bra Trylo Riza Bareneck Women Balconette Lightly Padded Bra - Buy
Trylo Riza Bareneck Women Balconette Lightly Padded Bra - Buy 2 Packs Women'S Silk Thermal Underwear Set Mulberry Silk Long Base Lay – Easyhot the hot clothing company
2 Packs Women'S Silk Thermal Underwear Set Mulberry Silk Long Base Lay – Easyhot the hot clothing company Workout at Home. Beautiful Woman`s Breasts. Big Boobs in the Gym Stock Photo - Image of exercise, breast: 213628466
Workout at Home. Beautiful Woman`s Breasts. Big Boobs in the Gym Stock Photo - Image of exercise, breast: 213628466 Bestcorse XL Seamless Plus Size Corset Dress Full Slip Camisole Underdress Body Shaper Dress With Bra Tummy Control Under Dress Slimming Abdominal Abdomen Belly Girdle Bodyshaper Slim Waist Shapewear For Women Dress
Bestcorse XL Seamless Plus Size Corset Dress Full Slip Camisole Underdress Body Shaper Dress With Bra Tummy Control Under Dress Slimming Abdominal Abdomen Belly Girdle Bodyshaper Slim Waist Shapewear For Women Dress Always Ready (TV series) - Wikipedia
Always Ready (TV series) - Wikipedia