The Cottrell Experiment and Diffusion Limitation 3/3 - Electrochemical Double Layer - PalmSens
4.9 (736) In stock

In this chapter the electrochemical double layer and its features are discussed. The electrochemical double layer acts as a capacitor and every change in the potential of the electrode will induce a capacitive charging current that is caused by physics not by a chemical reaction. This current decays exponentially.
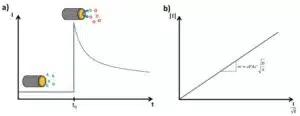
The Cottrell Experiment and Diffusion Limitation 2/3 - The

PDF) Finite Heterogeneous Rate Constants for the Electrochemical

Double layer (surface science) - Wikipedia
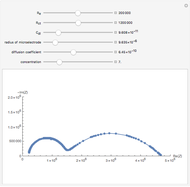
Cottrell Equation for the Potential-Step Experiment - Wolfram

Chronoamperometry - Electrochemistry Flashcards

Schematic representation of electrical double layer and the

Alternative representation of the Cottrell diffusion according to
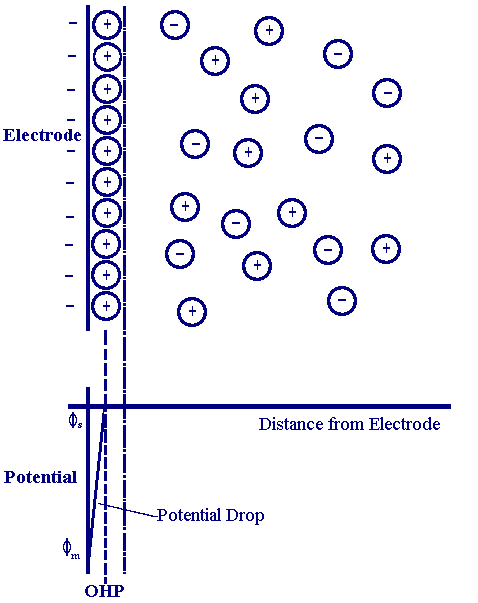
The Electrical Double Layer Department of Chemical Engineering

Model of electrical double layer on the positively charged

Electrochemical Impedance Spectroscopy (EIS) - PalmSens
Electric Double-Layer Capacitor (EDLC) How it works, Application & Advantages
Principles of EDLCs|TDK Techno Magazine|Electronics ABC|Learn about Technology with TDK
Electric-double-layer field-effect transistors with ionic liquids
Principles of EDLCs|TDK Techno Magazine|Electronics ABC|Learn
 Beyond Yoga All Deals, Sale & Clearance
Beyond Yoga All Deals, Sale & Clearance Lucky Brand Boxers for Men, Online Sale up to 59% off
Lucky Brand Boxers for Men, Online Sale up to 59% off Rule34 - If it exists, there is porn of it / matsunaga kouyou / 5850601
Rule34 - If it exists, there is porn of it / matsunaga kouyou / 5850601- SHEATH on Instagram: SHEATH x @UFC
 BOTA BOXEO EVERLAST ULTIMATE - Deportes Jimmy
BOTA BOXEO EVERLAST ULTIMATE - Deportes Jimmy Kid's Street Style Pocket Patched Cargo Pants Trendy Elastic - Temu
Kid's Street Style Pocket Patched Cargo Pants Trendy Elastic - Temu
