Compression Factor and Fugacity
5 (268) In stock

Share your videos with friends, family and the world

DOC) Computation of the Compression Factor and Fugacity Coefficient of Real Gases

EXER 2 FULL REPORT CHEM 111.1.docx - Name: Lanoline C. Toring Date Performed: February 7/14 2020 Section: 7L Date Submitted: February 21 2020 Group

PDF) Compressibility charts for gases with different acentric factors and evaluation of the Redlich-Kwong-Soave equation of state

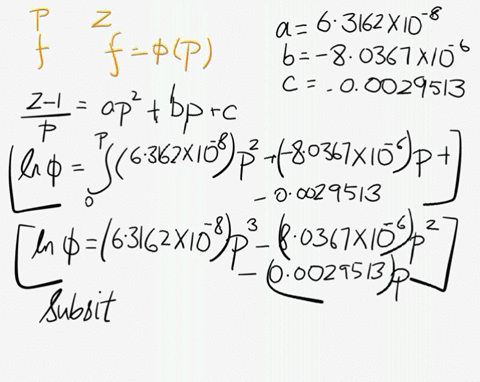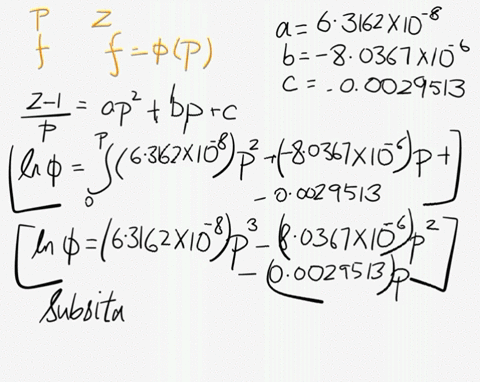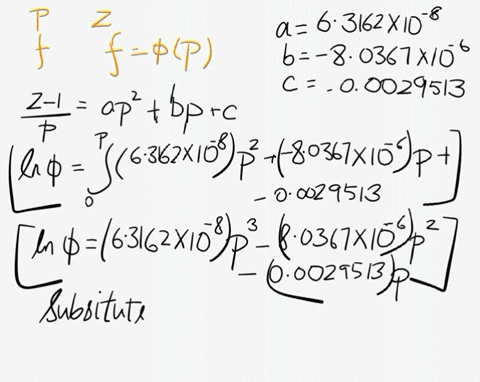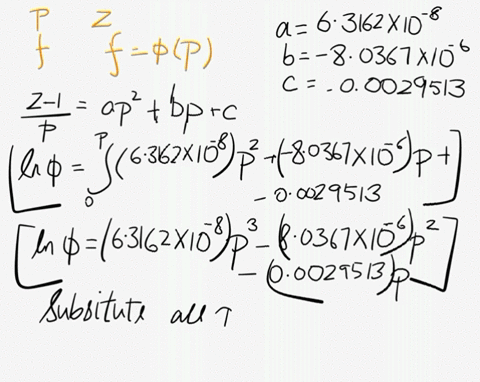
SOLVED: At 200 K, the compression factor of oxygen varies with pressure as shown below. Evaluate the fugacity of oxygen at this temperature and 100 atm: p (atm) Z 1,0000 0.9971 4.00000 0.98796 7,00000 0.97880 10,0000 0.96956 40.00 0.8734 70.00 0.7764

Molecules, Free Full-Text

Compression Factor and Fugacity

SOLVED: For a gas at a given temperature, the compression factor is described by the empirical equation: z = 1 - 8.50 × 10^(-3)P/P° + 3.50 × 10^(-5)(P/P°)^2 where P° = 1
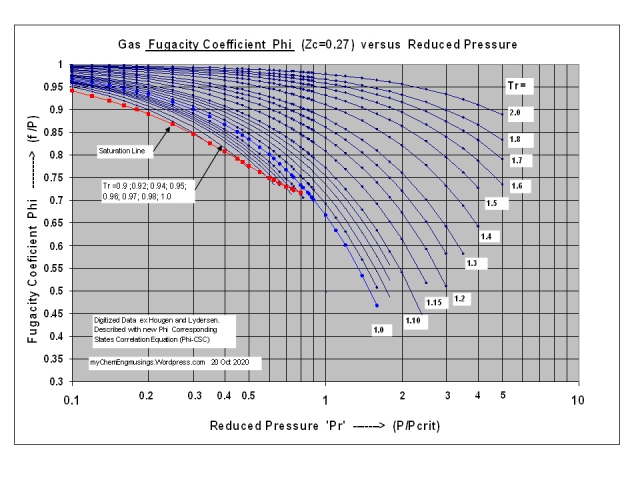
Two Simple yet Accurate Equations for Calculating the Fugacity Coefficient Phi and the Gas Compressibility Factor Z

CHEM111.1 - Exer2 Full-Report.docx - Name: Nicole Ann M. Pedrina Date Performed: Aug. 30 & Sept. 6 '18 Group No.: 1 Date Submitted: Sept. 14 '18 Section

CHEM 111 : Physical Chemistry - University of the Philippines Los Baños
Derive an expression for the compression factor of a gas tha
the compression factor one mole of a vander waals gas 0 C and 100 atm pressure is found to be 0.5
PPT - Real gases PowerPoint Presentation, free download - ID:3959491
Solved) - For values of z near 1, it is a good approximation to write z(P) = - (1 Answer)
What is the value of compression factor Z for the gas? (A) 1 (B) >1 (C) <1 (D) Zero
 Jil Sander Black Elasticized Waistband Shorts Jil Sander
Jil Sander Black Elasticized Waistband Shorts Jil Sander New Radiant Vanity Fair 3 pairs Hi-Cut Comfort Panties Womens
New Radiant Vanity Fair 3 pairs Hi-Cut Comfort Panties Womens Mono Blue Mill Deck - Very Strong - MTG Magic the Gathering deck - Pauper Legal!! Ready to Play
Mono Blue Mill Deck - Very Strong - MTG Magic the Gathering deck - Pauper Legal!! Ready to Play 20pcs Hard Wall Picture Frame Hooks Hangers 4-Pin White : Home & Kitchen
20pcs Hard Wall Picture Frame Hooks Hangers 4-Pin White : Home & Kitchen tag de Páscoa com dourado -hot stamping 20 unidades
tag de Páscoa com dourado -hot stamping 20 unidades- SKIMS + finds!✨ PRIME DAY shapewear + $19.99
