At low pressure, the van der waal's equation is written as (P+ a/V
4.6 (774) In stock
At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to :
At low pressure- the van der waal-s equation is written as -P- a-V-2-V-RT - Then compressibility factor is then equal to

Isotherms of van der Waals equation in reduced form, showing
Why does the van der Waals equation have one positive and one

SOLVED: I need the answer as soon as possible. 16. If Z is a

PQ) (v-b) = RT How it is written as P = Quez

Van Der Waals, PDF, Gases

At low pressures For 1 mole, the van der Waals equation is written

Van der Waals Equation of State in Python

At low pressure the van der Waals' equation is reduced to `[P +(a
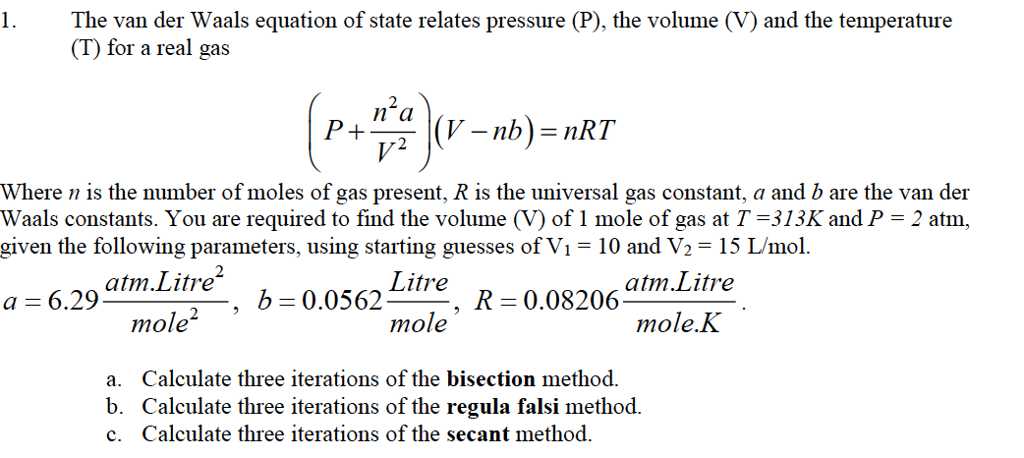
Solved 1.The van der Waals equation of state relates

Bengali] At a low pressure, the van der waals equation reduces to (P+

A Quick Guide on Van der Waals Equation
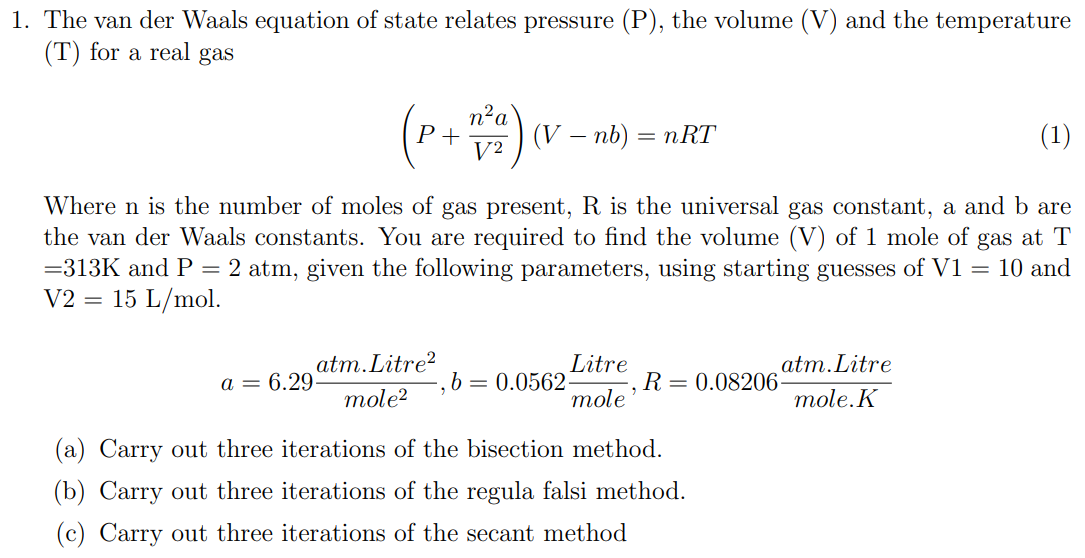
Solved The van der Waals equation of state relates pressure
Excel Calculations: Compressibility Factor Calculator for Excel
1. The compressibility factor, z, is the ratio of
Gas Compressibility Factor Calculator Excel SpreadsheetLow Cost
What is the compressibility factor (Z) for 0.02 mole of a van der
 Large Laundry Bag, Mesh Laundry Bags With Drawstring, Durable Wash Bag For Delicates, Garment Laundry Mesh Bag For Family, College Dorm, Apartment
Large Laundry Bag, Mesh Laundry Bags With Drawstring, Durable Wash Bag For Delicates, Garment Laundry Mesh Bag For Family, College Dorm, Apartment More is more: TikTok influencer Sara Camposarcone talks maximalist fashion, Arts & Design
More is more: TikTok influencer Sara Camposarcone talks maximalist fashion, Arts & Design Feelingirl Thong Shapewear Bodysuit for Women Tummy Control V
Feelingirl Thong Shapewear Bodysuit for Women Tummy Control V Women Sports Bra Push Up Fitness Bras One Shoulder Shockproof Yoga Bra Black White Yoga Running
Women Sports Bra Push Up Fitness Bras One Shoulder Shockproof Yoga Bra Black White Yoga Running Burgundy Flared Velvet Pants – Earth Gallery
Burgundy Flared Velvet Pants – Earth Gallery Sexy Women Breast Pads Silicone Bra Gel invisible inserts Push Up Bra Insert Breast Bra Cleavage
Sexy Women Breast Pads Silicone Bra Gel invisible inserts Push Up Bra Insert Breast Bra Cleavage