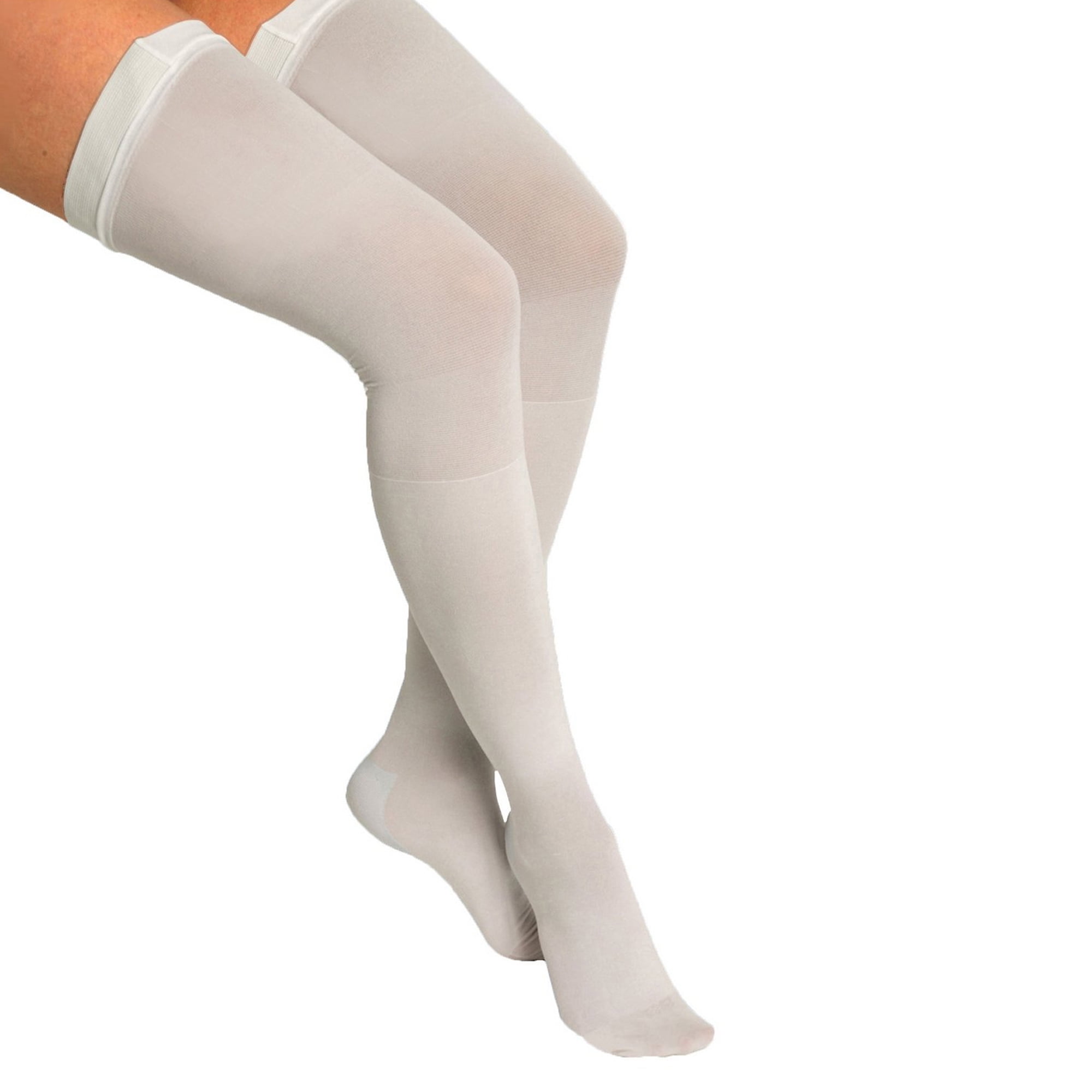
ITA-MED Style H-40 Lace Top Thigh Highs w/ Silicone Band
4.6 (783) In stock

Medium-strength graduated compression (20-22 mmHg) provides stronger support at the foot and ankle, where you need it most, and gradually decreases up
ITA-MED Unisex Anti-Embolism Thigh High Compression Stockings (18 mmHg): H-500

Thigh High Compression Stockings Mmhg

Knee High Nylons

Lace Top Thigh High with Back Seam

ITA-MED Thigh Highs (Open Toe) - Strong Compression 25-35 mmHg
Medium-strength graduated compression (20-22 mmHg) provides stronger support at the foot and ankle, where you need it most, and gradually decreases up

ITA-MED Style H-40 Lace Top Thigh Highs w/ Silicone Band

ITA-MED Sheer Thigh High Compression Stockings for Women (Lace Top w/Silicone Band) - Graduated Compression 20-22 mmHg, Closed Toe, Black, XX-Large : Health & Household

babyMaternity Awards
80% Nylon, 20% Spandex Imported HIGHLY RECOMMENDED BY DOCTORS: To help treat blood clots, varicose veins and other venous disorders. Ideal during and

ITA-MED Sheer Thigh High Compression Stockings for Women (Lace Top w/Silicone Band) - Graduated Compression 20-22 mmHg, Closed Toe, Black, Medium

ITA-MED Sheer Thigh High Compression Stockings for Women (Lace Top w/Silicone Band) - Graduated Compression 20-22 mmHg, Closed Toe, Beige, Large : Health & Household
Medium-strength graduated compression (20-22 mmHg) provides stronger support at the foot and ankle, where you need it most, and gradually decreases up

ITA-MED Style H-40 Lace Top Thigh Highs w/ Silicone Band

babyMaternity Awards

ITA-MED Sheer Thigh High Compression Stockings for Women (Lace Top w/Silicone Band) - Graduated Compression 20-22 mmHg, Closed Toe, Black, XX-Large : Health & Household
Energy Healing NY ITA Energy Medicine
De West Wind ita-med-advanced-post-op-fracture-walker-brace-fwb
Applications for Medical Device Investigational Testing Authorizations Guidance Document
 What Does It Mean If You Have Purple Period Blood?
What Does It Mean If You Have Purple Period Blood? Womens Ladies Sports Sleep Comfort Bras Full Cup Non-Wired Seamless Vest Tops - Helia Beer Co
Womens Ladies Sports Sleep Comfort Bras Full Cup Non-Wired Seamless Vest Tops - Helia Beer Co- Bluebella Emilia Glitter Strap Bandaged Thong
 Wool mohair pant in blue
Wool mohair pant in blue Cato Fashions Cato Plus Size Love Words Panty Set
Cato Fashions Cato Plus Size Love Words Panty Set Lace bandeau and hooks Large/Extra Large, Black, 1 unit – Secret : Underwear
Lace bandeau and hooks Large/Extra Large, Black, 1 unit – Secret : Underwear
